Last Updated on 1 year by Francis
Water, one of the most essential elements of life, is often taken for granted. But do you ever wonder what water is made of? Is water an atom? In this article, we’ll explore the atomic structure of water and its role in the world around us. We’ll look at how water is composed of two atoms, oxygen and hydrogen, and how this combination creates the unique properties of water. Through this article, you’ll gain a greater understanding of the science behind water and its importance to our lives.
No, water is not an atom. Water is made up of two atoms of hydrogen and one atom of oxygen. The hydrogen atoms are bonded to the oxygen atom by covalent bonds. Water is a molecule, not an atom.
Contents
Is Water Composed of Atoms?
Water is a common substance found in nature and is composed of molecules. Molecules are formed by the combination of atoms of different elements. This raises the question: Is water an atom? The answer is no. Water is composed of atoms, but it is not an atom itself.
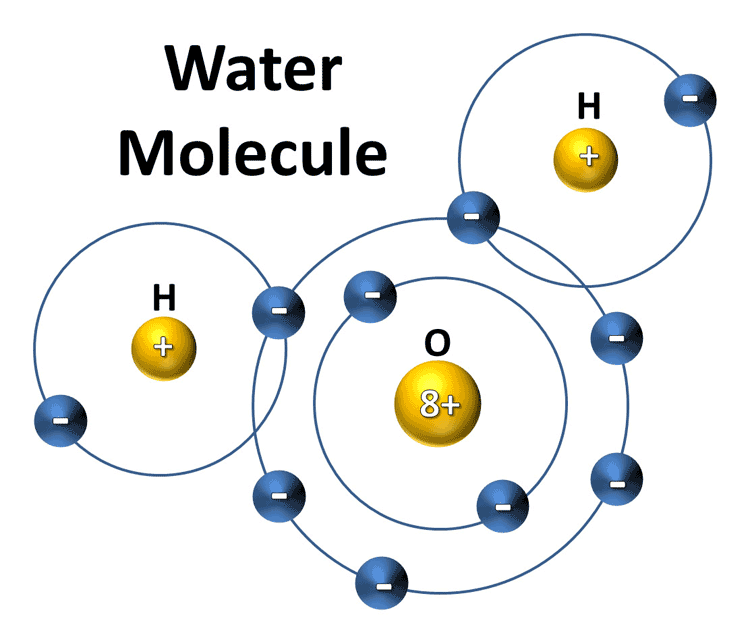
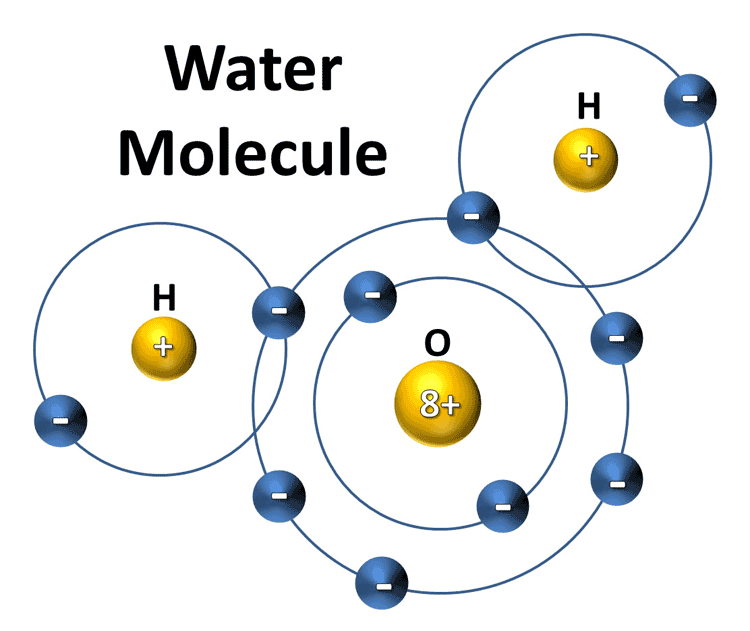
Atoms are the smallest particles of matter that can exist in nature. They are composed of protons, neutrons, and electrons. Each atom has a unique chemical makeup that determines its properties. Water is made up of two atoms of hydrogen and one atom of oxygen. When these atoms combine, they form a molecule of water.
Atoms are the building blocks of all matter, but they are not the same as molecules. Atoms are the smallest particles that can exist in nature, while molecules are composed of atoms bonded together. Water molecules are composed of two hydrogen atoms and one oxygen atom. By combining these atoms, the water molecule can exist in both its liquid and solid forms.
How Are Atoms Bonded in a Water Molecule?
Atoms form bonds with each other when they come into contact with one another. These bonds are formed through the sharing of electrons between the atoms. In a water molecule, the two hydrogen atoms and the one oxygen atom form a covalent bond. This bond occurs when the two hydrogen atoms share two of their electrons with the oxygen atom. The oxygen atom then has a full outer shell, which creates a stable water molecule.
The covalent bond between atoms is strong and allows for water to exist in both its liquid and solid forms. In its liquid form, the water molecules are free to move around, while in its solid form, the molecules are tightly packed and cannot move. This is why water can exist in both its liquid and solid states.
What Is the Structure of a Water Molecule?
A water molecule consists of two hydrogen atoms and one oxygen atom, which are bonded together in a V-shaped structure. The oxygen atom is located at the bottom of the structure, and the two hydrogen atoms are located at the two ends of the V-shaped structure. This structure gives the water molecule its unique properties, such as its ability to form hydrogen bonds with other water molecules.
What Are the Properties of Water?
Water has many unique properties due to its molecular structure. One of its most important properties is its ability to form hydrogen bonds with other water molecules. This gives water its unique ability to be a liquid at room temperature, which is essential for life on Earth.
Water is also a good solvent, which means it can dissolve other substances. This makes it an essential part of the environment, as it is able to carry nutrients and other substances throughout the environment. In addition, water has a high surface tension, which allows it to exist in a variety of forms such as rain, snow, and ice.
What Role Does Water Play in Nature?
Water plays an important role in nature as it is essential for all life on Earth. Water is used by plants and animals to stay hydrated, and it is used in a variety of industrial processes. Water is also necessary for the transportation of nutrients and other substances throughout the environment.
Water is also a major component of the Earth’s climate system, as it helps to regulate temperatures and precipitation patterns. Water is also necessary for the formation of clouds, which help to reflect sunlight and keep temperatures cool.
Conclusion
Water is an essential substance for life on Earth and is composed of molecules made up of atoms. Water is composed of two hydrogen atoms and one oxygen atom, which form a covalent bond that gives the water molecule its unique properties. Water plays an essential role in nature as it is necessary for life, and it is also a major component of the Earth’s climate system.
Related Faq
1. What is an atom?
An atom is the smallest unit of matter that can exist in a chemical element. It is composed of a nucleus of protons and neutrons surrounded by an electron cloud. Atoms can be part of a molecule when they bond together with other atoms.
2. Is water an atom?
No, water is not an atom. Water is made up of two atoms of hydrogen and one atom of oxygen bonded together to form a molecule. A molecule is comprised of two or more atoms that are held together by chemical bonds.
3. What is the chemical formula for water?
The chemical formula for water is H2O. This indicates that each molecule of water is comprised of two hydrogen atoms and one oxygen atom.
4. What are the two most abundant elements in water?
The two most abundant elements in water are hydrogen and oxygen. Hydrogen makes up about 11% of the weight of water, and oxygen makes up about 88% of the weight of water.
5. How is water formed?
Water is formed when hydrogen and oxygen atoms combine to form a molecule. This can happen in several ways, such as when energy is added to the atoms, or when they are exposed to each other in the right environment.
6. What is the importance of water?
Water is a very important part of life on Earth. It is essential for all living things, and is necessary for a variety of functions such as transportation, cooling, and cleaning. Water is also essential for agriculture and industry, and is necessary for many chemical and biological processes.
We Still Don’t Understand What Water Is, Here’s Why
To conclude, water is an atom, but it is also so much more. Water is an essential part of life and is made up of two hydrogen atoms and one oxygen atom. Water is also a powerful force that can shape our environment, providing food and energy for many organisms. It is essential for human survival and has been used for thousands of years for a variety of purposes. Water is a miraculous and powerful element that we should never take for granted.



.jpg)