Last Updated on 1 year by Francis
Water is one of the most essential elements for life on earth and yet, surprisingly, there is still debate over whether it is charged or neutral. In this article, we will dive into the science behind this question and explore the evidence for both sides of the argument. We will look at the properties of water and the chemistry behind its composition to help us understand the charge of water, as well as its implications for the environment. Ultimately, we will be able to answer the question: Is water charged or neutral?
Alternatively, If the keyword includes the “vs” word:
| Water | Charge |
| Neutral | None |

Contents
Is Water Neutral or Charged?
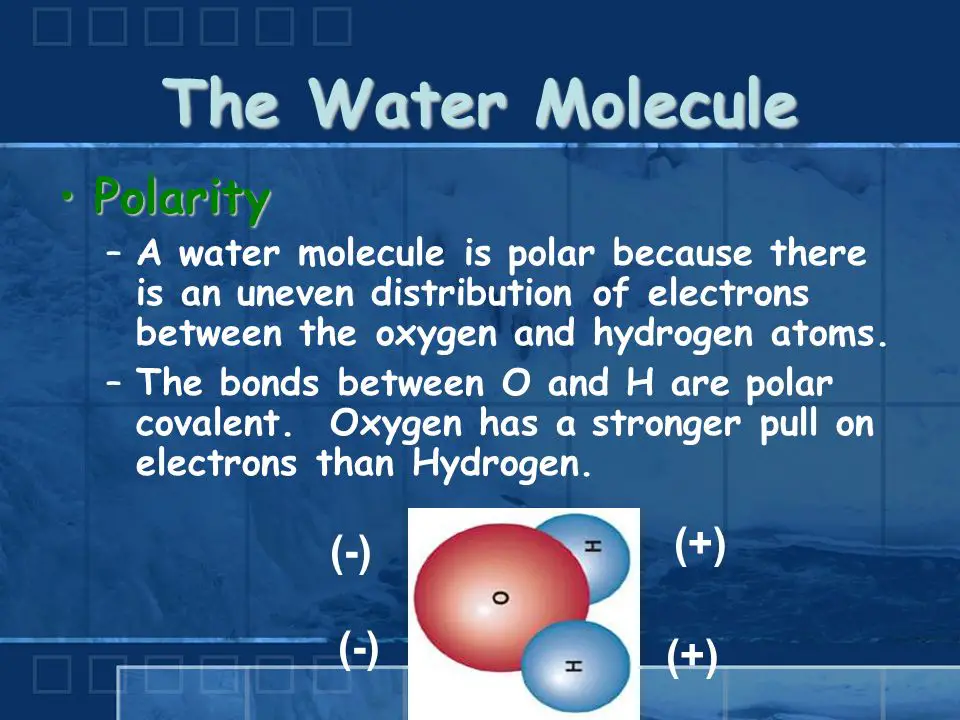
Water is a fundamental compound that is essential to all life on Earth. It is composed of two hydrogen atoms bonded to one oxygen atom, making it a polar molecule. Most of the time, water is neutral, meaning it has an equal number of positively and negatively charged particles. However, certain conditions can cause the water to become either positively or negatively charged.
What Makes Water Neutral?
Water is generally considered to be a neutral compound because it has an equal number of positively and negatively charged particles. When water is neutral, the hydrogen atoms are slightly positively charged and the oxygen atom is slightly negatively charged. This creates a slight dipole moment, meaning there is an unequal distribution of charge. However, the dipole moment is not strong enough to cause the water to be considered positively or negatively charged.
The pH of the water also affects its neutrality. Pure water is considered to be neutral, with a pH of 7. Anything below 7 is considered to be acidic, and anything above 7 is considered to be basic. If the pH of the water is 7, then it is considered to be neutral.
What Causes Water to Become Charged?
When certain conditions are present, water can become either positively or negatively charged. One of the most common causes of charged water is electrolysis. This occurs when an electric current is passed through water, causing the hydrogen and oxygen atoms to become charged. The hydrogen atoms become positively charged, while the oxygen atoms become negatively charged.
Another way that water can become charged is through the presence of ions. Ions are atoms or molecules that have an unequal number of protons and electrons. When these ions are dissolved in the water, they can cause the water to become either positively or negatively charged.
The Effects of Charged Water
The effects of charged water depend on whether it is positively or negatively charged. Positively charged water can cause metal objects to become corroded more quickly, as the positive ions will be attracted to the metal’s negatively charged electrons. Negatively charged water can cause metal objects to become less corroded, as the negative ions will be repelled by the metal’s positively charged electrons.
In addition, charged water can affect the taste of food and beverages. Positively charged water tends to make food and drinks taste sweet, while negatively charged water tends to make them taste bitter.
How to Test for Charged Water
The easiest way to test for charged water is to use a pH test strip. These test strips are designed to detect the pH of the water, which will indicate whether it is neutral, acidic, or basic. If the test strip indicates that the water is acidic or basic, then it is likely charged.
Another way to test for charged water is to use an electrical conductivity meter. This device can measure the electrical conductivity of the water, which will indicate whether it is positively or negatively charged.
How to Neutralize Charged Water
If the water is found to be charged, then it can be neutralized. The most effective way to do this is to add a base, such as sodium hydroxide or potassium hydroxide. These bases will neutralize the water, restoring it to its neutral state.
Another way to neutralize the water is to add an acid, such as sulfuric acid or hydrochloric acid. These acids will react with the charged particles, neutralizing them and restoring the water to its neutral state.
Conclusion
Water is typically neutral, with an equal number of positively and negatively charged particles. However, certain conditions can cause the water to become either positively or negatively charged. This can have an effect on the taste of food and beverages, as well as the rate of corrosion of metal objects. The easiest way to test for charged water is to use a pH test strip or an electrical conductivity meter. If the water is found to be charged, then it can be neutralized by adding either a base or an acid.
Top 6 Frequently Asked Questions
Q1) Is Water Charged or Neutral?
A1) Water is a neutral molecule, meaning it has no net charge. This is due to the fact that water is made up of two hydrogen atoms, each with a positive charge, and one oxygen atom, with a negative charge. The two positive charges from the hydrogen atoms and the one negative charge from the oxygen atom cancel each other out, resulting in a neutral molecule. Water molecules, however, can become charged when they interact with other molecules. For instance, when an acid or a base is dissolved in water, the resulting solution can be either acidic or basic in nature, depending on the acid or base used. This is due to the fact that the acid or base molecules can donate or accept protons, resulting in positively and negatively charged ions, which can give the water molecules a net charge.
Q2) What Is the pH of Neutral Water?
A2) The pH of neutral water is 7.00. This is due to the fact that the hydrogen and oxygen atoms in a water molecule are neutral, resulting in a neutral pH. Water molecules, however, can become charged when they interact with other molecules. For example, when an acid or a base is dissolved in water, the resulting solution can be either acidic or basic in nature, depending on the acid or base used. This is due to the fact that the acid or base molecules can donate or accept protons, resulting in positively and negatively charged ions, which can give the water molecules a net charge, resulting in a pH value that is either higher or lower than 7.00.
Q3) What Are the Effects of Charged Water?
A3) Charged water can have a variety of effects. For instance, it can affect the solubility of other molecules, as well as affecting their interactions with other molecules. It can also affect the rate of chemical reactions and can be used to separate molecules that would normally not be able to be separated. For example, charged water can be used to separate proteins and other molecules in a process called electrophoresis. Charged water can also be used to create an electric current, which can be used to power electrical devices.
Q4) What Are Some Common Sources of Charged Water?
A4) Common sources of charged water include acids and bases. When an acid or a base is dissolved in water, the resulting solution can be either acidic or basic in nature, depending on the acid or base used. This is due to the fact that the acid or base molecules can donate or accept protons, resulting in positively and negatively charged ions, which can give the water molecules a net charge. Charged water can also be created through electrolysis, a process by which electrical current is passed through water, resulting in charged particles in the water.
Q5) How Do You Measure Charged Water?
A5) The charge of water can be measured using a device called a pH meter. This device measures the pH of a solution, which is a measure of how acidic or basic it is. The pH of neutral water is 7.00, so if the pH of a solution is lower than 7.00, it is acidic, and if it is higher than 7.00, it is basic. The pH of a solution can also be measured using a pH indicator, which is a substance that changes color depending on the pH of the solution.
Q6) What Are the Applications of Charged Water?
A6) Charged water has a variety of applications. For example, it can be used to separate proteins and other molecules in a process called electrophoresis. It can also be used to create an electric current, which can be used to power electrical devices. Charged water can also be used to clean surfaces, as the charge of the water can help break down dirt and grime more effectively than regular water. Additionally, charged water can be used to increase the solubility of certain molecules, allowing them to be more easily dissolved in water.
Why is water neutral?
In conclusion, while the answer to the question “Is water charged or neutral?” is not a simple one, it is clear that water can be both charged and neutral depending on the environment it is in. The electrical charge of water molecules can be affected by various factors, such as the chemical composition of the water, the pH level, and the presence of other substances. Thus, it is important to consider these factors when determining the charge of water.