Last Updated on 1 year by Francis
Oxygen is one of the most important elements on our planet. It is essential to life, and without it, we wouldn’t be able to survive. But is oxygen a positive or negative ion? This is a question that has been debated for centuries, and it is still a subject of much controversy among scientists. In this article, we will explore the arguments for both sides of the debate and uncover the answer to the age-old question: Is oxygen a positive or negative ion?
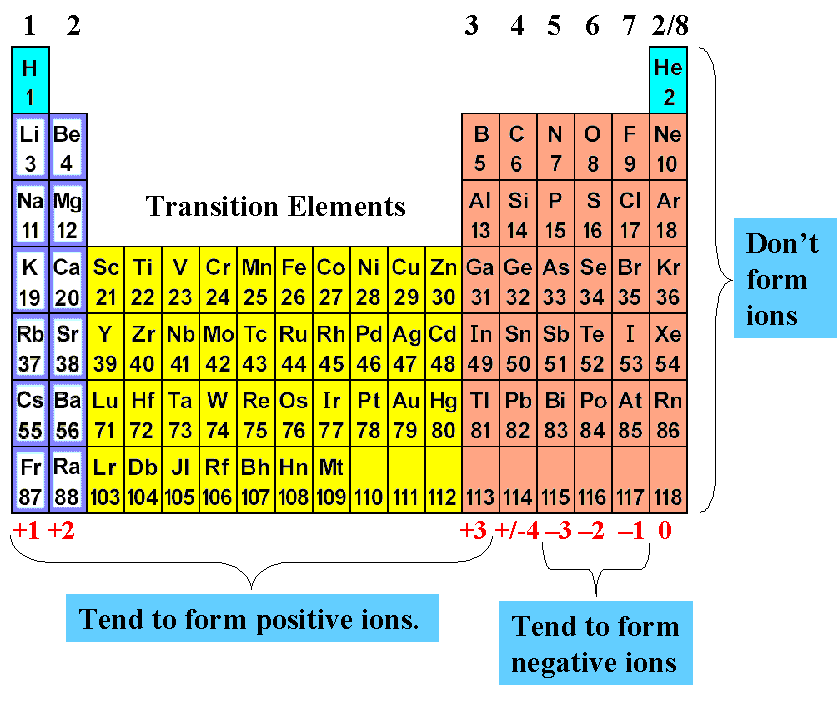
Oxygen is a neutral non-metal element and does not form any ions. Therefore, Oxygen does not have a positive or negative charge.

Contents
Oxygen as a Negative Ion
Oxygen is a negative ion, meaning it has one or more extra electrons in its outer electron shell. This extra electron makes it unstable, so it seeks to bond with other elements. Oxygen is the most abundant element in the Earth’s atmosphere, and it plays an important role in the formation of many minerals. The negative charge of oxygen is also responsible for its ability to form strong bonds with other elements, such as hydrogen and carbon.
Oxygen is also a key component of many acids, such as sulfuric acid and hydrochloric acid. These acids are formed when oxygen combines with other elements, such as hydrogen or carbon, to form compounds. The negative charge of oxygen makes it easier for these compounds to react with other molecules, resulting in the formation of acids.
Oxygen is also an important part of many organic molecules, such as proteins and enzymes. Proteins are made up of amino acids, which are molecules that contain both an oxygen atom and a carbon atom. Enzymes are proteins that catalyze chemical reactions, and they contain a cofactor, which is an oxygen-containing molecule. The negative charge of oxygen makes it easier for these molecules to interact with other molecules and catalyze the reactions they need to function.
Oxygen’s Role In Oxidation Reactions
Oxygen is also involved in a type of chemical reaction known as oxidation. During oxidation, oxygen combines with another element, such as hydrogen or carbon, to form an oxide. This reaction releases energy, which can be used for various purposes. For example, oxidation reactions are responsible for the combustion of fuels, such as gasoline. The burning of gasoline releases energy, which is then used to power cars and other machines.
Oxidation reactions are also involved in the corrosion of metals, such as iron and steel. When iron and steel are exposed to oxygen, they form oxides, which cause them to corrode. The oxidation of iron and steel is responsible for the rust that is commonly seen on these metals.
Oxygen’s Role In Photosynthesis
Oxygen also plays an important role in photosynthesis, which is the process by which plants convert sunlight into energy. During photosynthesis, plants absorb carbon dioxide from the air and combine it with water, creating glucose and releasing oxygen as a byproduct. This oxygen is then released into the atmosphere, where it can be used by animals and humans to breathe.
Oxygen as a Positive Ion
Oxygen can also exist as a positive ion, meaning it has one less electron in its outer electron shell. When oxygen is in this state, it is said to be “oxidized.” This state can occur when oxygen reacts with another element, such as hydrogen, to form an oxide. The positive charge of oxygen makes it easier for it to react with other elements, resulting in oxidation reactions.
Positively charged oxygen atoms can also form strong bonds with other elements, such as metals. This is why many metals, such as iron and steel, are prone to rusting, as oxygen atoms attach themselves to the metal and form oxides. This oxidation can lead to corrosion, which is the weakening and eventual destruction of metals.
Oxygen’s Role In Combustion Reactions
Positively charged oxygen atoms can also play a role in combustion reactions, which are responsible for the burning of fuels, such as gasoline. During combustion, oxygen atoms react with the fuel molecules, releasing energy in the form of heat and light. This energy is then used to power cars and other machines.
Oxygen’s Role In Respiration
Oxygen is also an important component of respiration, which is the process by which animals and humans extract energy from food. During respiration, oxygen is combined with glucose, releasing energy in the form of ATP molecules. This energy is then used to power the body’s cells, allowing them to function properly.
Without oxygen, respiration would not be possible, and animals and humans would not be able to survive. This is why it is so important for us to have access to clean air, which is rich in oxygen.
Few Frequently Asked Questions
What is an Ion?
An ion is an atom or molecule that has gained or lost one or more electrons, giving it a net positive or negative electrical charge. Ions are formed when a neutral atom or molecule gains or loses one or more electrons, creating a charged particle.
Is Oxygen a Positive or Negative Ion?
Oxygen is a neutral atom; it does not carry a net electrical charge. When it forms an ion, it either gains two electrons to become an oxide ion (O2-) or loses two electrons to become an oxide cation (O2+). Therefore, oxygen can be either a positive or negative ion, depending on the number of electrons it has.
What is the Oxidation State of Oxygen?
The oxidation state of oxygen is typically -2, since it has two negative electrons. This means that oxygen is most often found as an anion, or a negatively charged ion, when it forms an ionic compound.
How Does Oxygen Form an Ion?
Oxygen can form an ion in two ways. If it gains two electrons, it becomes an oxide ion (O2-); if it loses two electrons, it becomes an oxide cation (O2+). In both cases, the oxygen atom has a net electrical charge, either positive or negative.
What is an Oxide Ion?
An oxide ion is an ionic compound in which an oxygen atom has gained two electrons. It carries a net negative charge and is the most common form of oxygen when it forms an ion.
What is an Oxide Cation?
An oxide cation is an ionic compound in which an oxygen atom has lost two electrons. It carries a net positive charge and is the second most common form of oxygen when it forms an ion.
Boost Your Mood With Negative Ions
In conclusion, Oxygen is neither a positive or negative ion, but instead, it is a neutral molecule. Oxygen plays an integral part in many chemical reactions and is essential for life on Earth. Without it, there would be no life. Without further research, it is impossible to understand the full scope of the role oxygen plays in our universe. Therefore, it is essential that we continue to research this element in order to understand its true potential.


.jpg)
.jpg)