Last Updated on 1 year by Francis
Alcohol is a common substance that many of us consume on a regular basis, but is it a polar substance? This question has been debated for decades and this article will discuss whether or not alcohol is truly polar in nature. We will examine the molecular structure of alcohol, its interaction with other substances, and how it behaves in different environments to see if it is a polar or non-polar molecule. By the end of the article, we should have a definitive answer as to whether or not alcohol is a polar substance.
Contents
What is a Polar Substance?
A polar substance is a compound or molecule that has a positive and negative end, similar to a magnet. This is due to the uneven distribution of electrons. Polar substances are generally considered to be more soluble in water than non-polar substances.
In order for a compound to be classified as polar, it must have an asymmetrical molecular geometry. This means that the positive and negative charges are not evenly distributed across the molecule. This can be caused by the presence of an electronegative atom, such as oxygen, nitrogen, or sulfur. Polar molecules will often form hydrogen bonds with other polar molecules, allowing them to dissolve in water.
What is Alcohol?
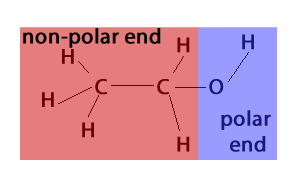
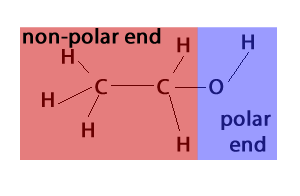
Alcohol is an organic compound that contains an oxygen atom, which makes it polar. Alcohols are classified as either straight-chain or branched-chain molecules. Straight-chain alcohols will have a linear structure and are typically more soluble in water than branched-chain alcohols.
The oxygen atom in the alcohol molecule is electronegative, meaning it will attract electrons. This creates a partial negative charge around the oxygen atom, and a partial positive charge around the hydrogen atoms. This uneven distribution of electrons makes alcohol a polar molecule.
What are the Properties of Alcohol?
Due to its polarity, alcohol is highly soluble in water. This means that it is able to form hydrogen bonds with water molecules, allowing it to dissolve and mix with water. This property makes alcohol useful in a variety of applications, from cleaning and disinfecting to fuel and solvents.
Alcohol is also highly flammable and has a low boiling point. This makes it useful as a fuel and as a solvent for other compounds. Alcohol is also volatile and can easily evaporate, making it useful for extracting volatile compounds.
Is Alcohol a Polar Substance?
Yes, alcohol is a polar substance. The oxygen atom in the alcohol molecule is electronegative, meaning it attracts electrons and creates a partial negative charge around the oxygen atom. This uneven distribution of charges makes alcohol a polar molecule.
Due to its polarity, alcohol is highly soluble in water. This allows it to form hydrogen bonds with water molecules, allowing it to dissolve and mix with the water. This property makes alcohol useful in a variety of applications, from cleaning and disinfecting to fuel and solvents.
What is the Difference Between Alcohol and Non-Polar Compounds?
The main difference between alcohol and non-polar compounds is the presence of an electronegative atom. Alcohol contains an oxygen atom, which is electronegative and creates a partial negative charge around the oxygen atom. This uneven distribution of charges makes alcohol a polar molecule.
Non-polar compounds, on the other hand, do not contain electronegative atoms and have an even distribution of charges. This means that they are not soluble in water and are not as volatile as alcohol. Non-polar compounds are also not as flammable as alcohol.
What are the Uses of Alcohol?
Due to its properties, alcohol has a variety of uses. It is commonly used as a fuel, as a solvent for other compounds, and as a disinfectant. It is also used in the production of pharmaceuticals, perfumes, and other products, and as an ingredient in foods and beverages.
Few Frequently Asked Questions
What is alcohol?
Alcohol is an organic compound with the molecular formula C2H6O. It is a volatile, flammable, colorless liquid with a characteristic odor and burning taste. It is most commonly consumed as an intoxicant, a recreational drug, and a preservative. The consumption of alcohol is a social and cultural phenomenon, and its consumption is regulated in many countries.
What does polar mean?
Polar molecules are molecules with a difference in the distribution of electric charge between one end and the other. Polar molecules have a net dipole moment, which means that the molecule has a positive charge at one end and a negative charge at the other end. As a result, polar molecules are attracted to one another, and can form strong intermolecular bonds.
Is alcohol a polar molecule?
Yes, alcohol is a polar molecule. The oxygen atom in the alcohol molecule is more electronegative than the hydrogen and carbon atoms, which causes the oxygen atom to pull the electrons away from the hydrogen and carbon atoms. This creates a net dipole moment, making alcohol a polar molecule.
What are the effects of alcohol’s polarity?
The polarity of alcohol has several effects. First, it allows alcohol to form strong bonds with other polar molecules, such as water. This is why alcohol is soluble in water. Additionally, the polarity of alcohol makes it more volatile, which is why it has a distinct smell and taste. Finally, the polarity of alcohol makes it easier for the human body to absorb, which is why it has intoxicating effects.
What other molecules are polar?
Many other molecules are polar. Examples include acetic acid, formic acid, glycerol, and urea. These molecules all have a net dipole moment, meaning that the electrons are not evenly distributed throughout the molecule. This causes the molecule to be attracted to other molecules with similar dipole moments.
What are the dangers of consuming too much alcohol?
Consuming too much alcohol can have serious health consequences. Excessive alcohol consumption can lead to liver damage, heart disease, and stroke. Additionally, it can increase the risk of certain types of cancer, impair cognitive function, and increase the risk of accidents and injuries. It is important to drink responsibly and be aware of the risks associated with alcohol consumption.
Why is alcohol more polar than water?
In conclusion, it can be said that alcohol is certainly a polarizing topic. It has both positive and negative effects, and it is up to each individual to decide how they want to approach it. Some people may choose to completely abstain from alcohol, while others may choose to enjoy it in moderation. Ultimately, everyone must make the decision that best suits their lifestyle and personal beliefs.