Have you ever wondered what makes vinegar so tart? Vinegar is a common kitchen condiment that has been around for centuries, and its signature taste is due to the presence of acetic acid. But what percentage of acetic acid is actually in vinegar? In this article, we will explore what acetic acid is, how it is measured, and what percentage of acetic acid is found in vinegar.
The percentage of acetic acid in vinegar typically ranges from 4 to 8 percent. The amount will depend on the type of vinegar being used. White vinegar, for example, usually contains 5 to 8 percent acetic acid, while apple cider vinegar can range from 4 to 6 percent. Balsamic vinegar is usually the least acidic, with a range of 3 to 4 percent.

Contents
What is the Concentration of Acetic Acid in Vinegar?
Vinegar is a popular condiment and household cleaning agent that has been used for centuries. It is made by fermenting ethanol with acetic acid bacteria which produces acetic acid. The concentration of acetic acid in vinegar is usually between 4-8%, with some varieties reaching up to 18%. This makes vinegar an effective ingredient for a variety of uses, including cooking, cleaning, and preserving food.
The concentration of acetic acid in vinegar can vary depending on the type of vinegar and how it is made. For instance, white vinegar is typically made from distilled ethanol and can have a concentration of 5-7%. On the other hand, apple cider vinegar is made from fermented apple cider and can have a concentration of up to 18%. Additionally, some vinegars, such as balsamic, are aged for extended periods of time, which can increase the concentration of acetic acid.
When using vinegar for cooking, it is important to understand the concentration of acetic acid in the product. If the vinegar is too acidic, it can overpower the dish and make it unpleasant to eat. On the other hand, if the vinegar is not acidic enough, it may not have the desired flavor or effects. For this reason, it is important to check the label of the product to ensure that it contains the correct concentration of acetic acid.
How is Acetic Acid Concentration Measured in Vinegar?
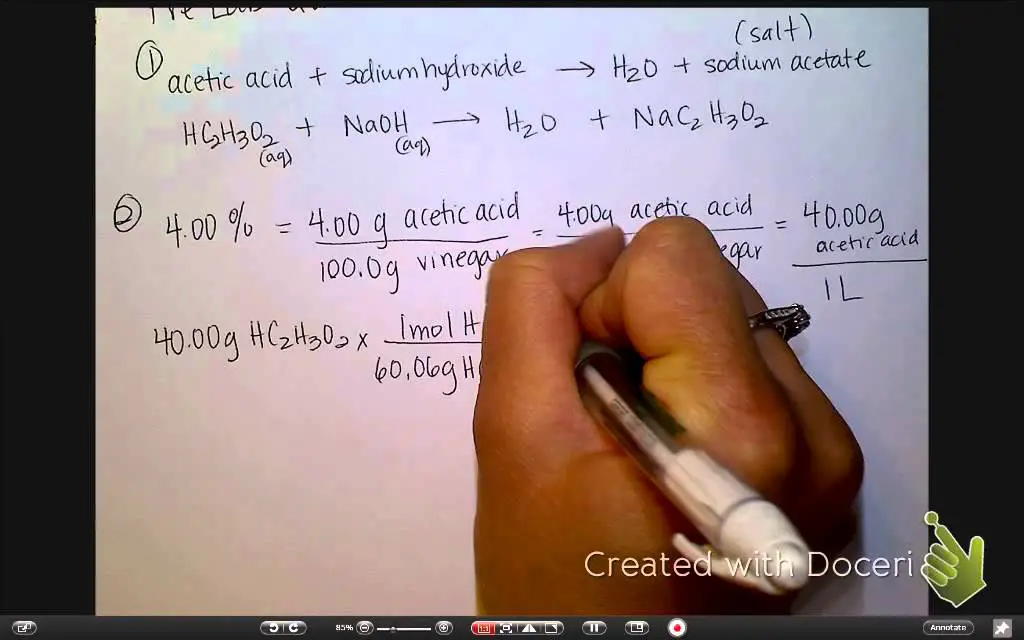
The concentration of acetic acid in vinegar is typically measured in terms of “percent acetic acid.” This is a measure of how much acetic acid is present in the vinegar relative to other ingredients. For example, a vinegar that contains 5% acetic acid means that 5% of the total volume of the vinegar is acetic acid.
The concentration of acetic acid in vinegar can be measured by a variety of methods. The most common method is titration, which involves adding a chemical reagent to a sample of vinegar and measuring the amount of acidity in the sample. Other methods include spectrophotometry, which uses light to measure the amount of acetic acid in the sample, and gas chromatography, which separates the components of the sample and measures the concentration of acetic acid.
Titration
Titration is a common method of measuring acetic acid concentration in vinegar. In this method, a known amount of chemical reagent is added to a sample of vinegar and the acidity of the sample is measured. The concentration of acetic acid is calculated based on the amount of reagent that is required to neutralize the acidity of the sample.
Titration is a simple and accurate way to measure the concentration of acetic acid in vinegar. However, it requires a calibrated burette and other specialized equipment, so it is not always practical for home use.
Spectrophotometry
Spectrophotometry is another method of measuring acetic acid concentration in vinegar. In this method, a sample of vinegar is illuminated with light of a specific wavelength. The amount of light that is absorbed by the sample is then measured and used to calculate the concentration of acetic acid.
Spectrophotometry is a quick and easy method of measuring acetic acid concentration in vinegar. However, it may not be as accurate as titration, and it requires specialized equipment.
How to Use Vinegar Safely?
Vinegar is a powerful ingredient that can be used for a variety of purposes. However, it is important to use it safely in order to avoid potential health risks.
Cooking
When using vinegar for cooking, it is important to use the correct concentration of acetic acid. Too much acetic acid can overpower the dish and make it unpleasant to eat. It is also important to use food-grade vinegar, as other types may contain contaminants that could be harmful.
Cleaning
Vinegar is a popular cleaning agent due to its acidic properties. However, it is important to use the correct concentration of acetic acid when cleaning. If the concentration is too high, it could cause damage to surfaces or skin. It is also important to dilute the vinegar with water before use, as undiluted vinegar can be too harsh for some surfaces.
Related Faq
What is Acetic Acid?
Acetic acid is a clear, colorless liquid with a strong, pungent odor and sour taste. It is a type of carboxylic acid, which contains a double-bonded oxygen and carbon atom in its molecular structure. It is a weak acid, meaning that it does not completely dissociate in water, which means the pH of a solution of acetic acid is usually between 2.4 and 3.4. Acetic acid can be found in many foods and household items, and is the main component of vinegar.
What is the Chemical Formula of Acetic Acid?
The chemical formula of acetic acid is C2H4O2. It has two atoms of carbon (C), four atoms of hydrogen (H), and two atoms of oxygen (O).
What is Vinegar?
Vinegar is a liquid that is made by fermenting any type of food source with bacteria and yeast, which produces acetic acid. Vinegar can be made from many types of food sources, including apples, grapes, grain, and potatoes. It is used in many cooking and cleaning applications, and can also be used as a natural preservative.
What is the Percentage of Acetic Acid in Vinegar?
The percentage of acetic acid in vinegar can vary depending on the type of vinegar and how it was made. Generally, the acetic acid content of vinegar will range anywhere from 4-8%. White vinegar typically has the highest percentage of acetic acid, ranging from 5-6%.
What other Acids are Present in Vinegar?
In addition to acetic acid, vinegar may also contain other acids such as lactic, malic, tartaric, citric, and formic acid, depending on the type of vinegar and how it was made. These additional acids can contribute to the flavor and aroma of vinegar, and can also affect its pH.
How is Acetic Acid Used?
Acetic acid is used in many applications, including food production, pharmaceuticals, and industrial processes. In food production, acetic acid is used to adjust the pH of foods and beverages, to add flavor, and to inhibit the growth of microorganisms. In pharmaceuticals, acetic acid is used in the production of drugs and as a preservative. In industrial processes, acetic acid is used as a solvent and in the manufacture of plastics, fibers, and other materials.
In conclusion, acetic acid is the main component of vinegar, making up between 4 and 8 percent of its total composition. This percentage can vary depending on the type of vinegar and how it has been processed. Regardless, it is the acetic acid present in vinegar that gives it its unique taste and odor.

.jpg)


.jpg)


