Last Updated on 1 year by Francis
Oxygen is essential for life, but do you know if it has a positive or negative charge? The answer may surprise you. In this article, we’ll explore the answer to the question: Is oxygen positive or negative? We’ll look at why it has a particular charge, the implications of that charge and what it all means for us. So read on to discover the truth about oxygen’s charge.
Oxygen is neither a positive nor a negative charge. It is neutral. Oxygen is an element found in the air and in many other places. It is a colorless, odorless gas that is essential for life on Earth. Oxygen is the most abundant element found in the Earth’s atmosphere, making up about 21% of the air. It is also found in water and in many other compounds. Oxygen is a very reactive element, which means that it readily combines with other elements to form compounds. It is also used in many industrial processes and is a key component of fuels and explosives.

Contents
What is the Nature of Oxygen?
Oxygen is an element found in the periodic table and is considered a non-metal. It is the most abundant element in the Earth’s atmosphere, comprising about 20 percent of the air we breathe. Oxygen is colorless, odorless, and tasteless, and it is found in the form of two atoms of oxygen bonded together. Oxygen is a highly reactive element, which means that it easily combines with other elements to form compounds. Oxygen is essential for all forms of life, and it is an important component of the Earth’s atmosphere.
Oxygen is an important component in many chemical reactions, and it has a variety of uses in industry and medicine. Oxygen is used in the production of steel, chemicals, plastics, and other materials. In medicine, oxygen is used to treat respiratory illnesses and to help patients with breathing problems. Oxygen is also used in welding, firefighting, and other hazardous occupations.
Is Oxygen Positive or Negative?
Oxygen is neither positive nor negative in terms of its electrical charge. It is considered to be neutral, meaning that it has no electrical charge at all. This is because oxygen has an equal number of protons and electrons, which results in a neutral charge. Oxygen atoms can still be attracted to positive and negative charges, however, and this attraction can be used to form chemical bonds.
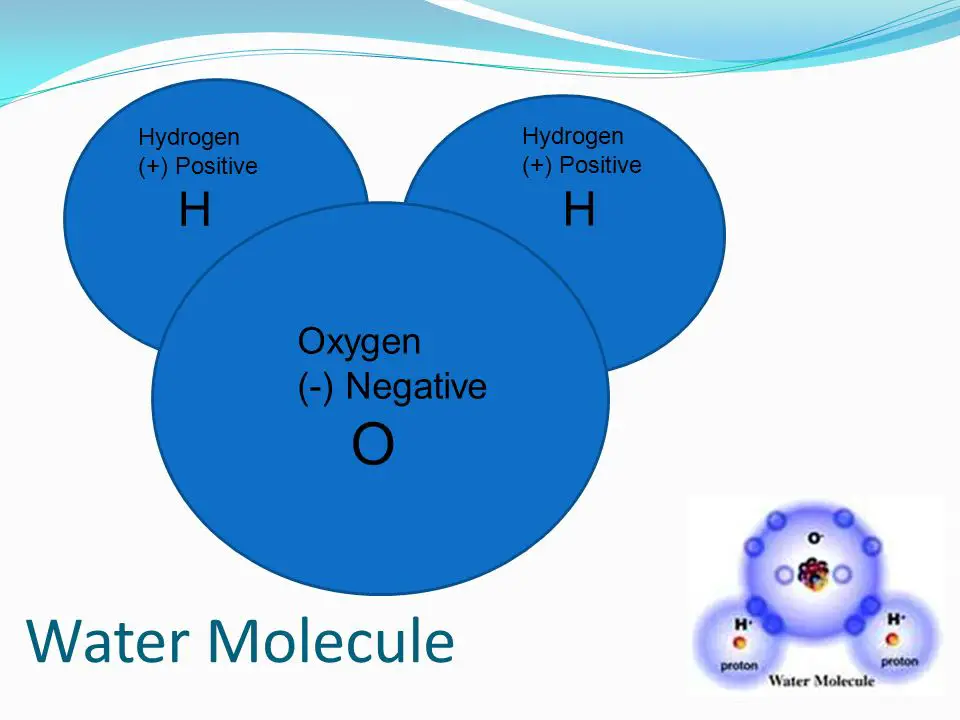
Although oxygen is neutral, the compounds that it forms can be either positively or negatively charged. For example, when oxygen combines with hydrogen to form water (H2O), the oxygen atom has a slightly negative charge and the hydrogen atoms have a slightly positive charge. This allows the oxygen and hydrogen atoms to form a bond, which is the basis of water molecules.
How Does Oxygen React with Other Elements?
Oxygen is a highly reactive element, meaning that it forms strong bonds with other elements. For example, when oxygen combines with carbon, it forms carbon dioxide (CO2), which is a major component of the Earth’s atmosphere. Oxygen also combines with nitrogen to form nitrous oxide (N2O), which is used in many industrial processes.
Oxygen is also used in the production of acids, including sulfuric acid (H2SO4). Oxygen is also used in combustion reactions, such as the burning of gasoline. In these reactions, oxygen combines with hydrocarbons to form carbon dioxide, water, and energy.
What Are the Properties of Oxygen?
Oxygen is a colorless, odorless, and tasteless gas at room temperature. It is found in the form of two atoms of oxygen bonded together. Oxygen is a highly reactive element, which means that it easily combines with other elements to form compounds. Oxygen is a necessary component of the Earth’s atmosphere, and it is essential for all forms of life.
Oxygen is a non-metal, meaning that it does not conduct electricity. It has a high electronegativity, meaning that it is able to attract electrons to itself. This makes it an ideal component for covalent bonds. Oxygen is also used in the production of many acids, including sulfuric acid.
Few Frequently Asked Questions
Question 1: Is Oxygen Positive or Negative?
Answer: Oxygen is neither positively nor negatively charged. Oxygen atoms have 8 protons in the nucleus and 8 electrons surrounding the nucleus. Since the number of protons and electrons are equal, the net electrical charge of an oxygen atom is neutral. This means that an oxygen atom is neither positively nor negatively charged.
Question 2: How is Oxygen Neutral?
Answer: Oxygen atoms have 8 protons in the nucleus and 8 electrons surrounding the nucleus. Since the number of protons and electrons are equal, the net electrical charge of an oxygen atom is neutral. This is because the protons carry a positive charge while the electrons carry a negative charge and the two cancel each other out.
Question 3: Does Oxygen Have a Magnetic Field?
Answer: Oxygen does not have a magnetic field. Even though oxygen atoms have an uneven distribution of electrons, which causes them to be slightly dipolar, the overall magnetic field of the atom is zero. This is because the electron spins and their associated magnetic fields cancel each other out.
Question 4: What is the Chemical Symbol for Oxygen?
Answer: The chemical symbol for oxygen is O. This is an abbreviation of the element’s full name, oxygenium. This symbol is used to represent oxygen atoms in chemical equations and formulas.
Question 5: Does Oxygen have Valence Electrons?
Answer: Yes, oxygen atoms have valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. Oxygen atoms have 8 electrons in the outermost shell and these are the atoms valence electrons.
Question 6: What is the Atomic Number of Oxygen?
Answer: The atomic number of oxygen is 8. This is the number of protons present in the nucleus of an oxygen atom. This is also the number of electrons present in the atom. The atomic number is used to identify different elements and is a key factor in determining the properties of an element.
Positive and Negative Charge
In conclusion, oxygen is one of the most important elements on the planet and its charge is neither positive or negative. Instead, it is a neutral element that is essential for life to exist, making it an invaluable element for all life forms. As such, oxygen should be properly valued and respected for its fundamental role in the environment.