Last Updated on 1 year by Francis
Isopropyl alcohol is a common household cleaning agent that has a wide range of uses, from cleaning surfaces to disinfecting wounds. But have you ever wondered how long it takes for isopropyl alcohol to evaporate? In this article, we will be exploring the answer to this question and discussing the factors that can affect the evaporation rate of isopropyl alcohol. From the surface area of the liquid to the temperature of the environment, we’ll look at the science behind isopropyl alcohol’s evaporation and the best way to store it. So, if you’re curious about the evaporation rate of isopropyl alcohol, read on!
Isopropyl alcohol evaporates quickly, in about the same time as water. Generally, the evaporation time for a liquid solvent is greater if it has a higher molecular weight and boiling point. The evaporation time for isopropyl alcohol is approximately two to five minutes.

Contents
How Long Does Isopropyl Alcohol Take to Evaporate?
Isopropyl alcohol, also known as rubbing alcohol, is a common household product that is often used to clean wounds, sterilize surfaces, and remove sticky residue from objects. It is also used as an ingredient in some cleaning products. While it is a convenient and effective cleaning agent, it evaporates quickly, leaving behind no residue. In this article, we will explore how long it takes for isopropyl alcohol to evaporate.
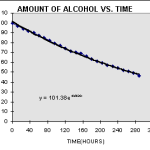
Isopropyl alcohol is a volatile compound, meaning that it evaporates quickly when exposed to the air. The evaporation process is accelerated by heat and air circulation. At room temperature, isopropyl alcohol takes about 10 minutes to evaporate completely. However, the evaporation rate can be affected by other factors such as humidity and the concentration of the isopropyl alcohol solution. For example, when the solution is more concentrated, it will take longer to evaporate.
Factors That Affect the Evaporation Rate of Isopropyl Alcohol
The rate at which isopropyl alcohol evaporates is affected by several factors, such as the concentration of the solution, temperature, air circulation, and humidity. The higher the concentration of the isopropyl alcohol solution, the longer it will take to evaporate. The higher the temperature, the faster the evaporation rate. Additionally, air circulation can help to speed up the evaporation process. Finally, higher humidity levels can slow down the rate at which the isopropyl alcohol evaporates.
Uses for Isopropyl Alcohol
Isopropyl alcohol is a versatile cleaning agent that can be used for a variety of purposes. It is often used to clean and sterilize surfaces, remove sticky residue from objects, and clean wounds. It is also an effective disinfectant and can be used to kill germs, bacteria, and other microorganisms. Isopropyl alcohol can also be used as an ingredient in some cleaning products, such as window and glass cleaners.
Safety Considerations When Using Isopropyl Alcohol
Isopropyl alcohol is flammable and should be used with caution. It should be kept away from open flames and other sources of heat. Isopropyl alcohol should also be kept out of reach of children and pets. When using isopropyl alcohol to clean wounds, it should be applied with a cotton swab and the excess should be blotted off with a clean cloth.
How to Store Isopropyl Alcohol
Isopropyl alcohol should be stored in a cool, dry place and in a tightly sealed container. It should be kept away from sources of heat or flame and out of reach of children and pets. Isopropyl alcohol should also be kept away from other flammable materials such as paint thinners or gasoline.
Alternatives to Isopropyl Alcohol
Isopropyl alcohol is an effective cleaning agent but it is not the only one available. Other alternatives include white vinegar, hydrogen peroxide, and rubbing alcohol. Each of these cleaning agents has its own advantages and disadvantages. For example, white vinegar is a natural cleaning agent and is less toxic than isopropyl alcohol, but it is not as effective at killing germs and bacteria. Hydrogen peroxide is an effective disinfectant but it can damage certain surfaces. Rubbing alcohol is a less abrasive alternative to isopropyl alcohol but it is not as effective at removing sticky residue.
Frequently Asked Questions
Question 1: What is Isopropyl Alcohol?
Answer: Isopropyl alcohol, commonly referred to as IPA or isopropanol, is a colorless, flammable chemical compound with a strong odor. It is a type of alcohol, similar to ethanol but with different properties and uses. It is a common ingredient in many household and industrial cleaning products, and is also used as a solvent in the production of medicines, perfumes, and dyes.
Question 2: Why Is Isopropyl Alcohol Used?
Answer: Isopropyl alcohol is a multi-purpose solvent and cleaning agent that can be used in a variety of applications. It is often used as a cleaning agent to remove dirt, grease, and other materials from surfaces. It is also used in the production of medicines, perfumes, dyes, and other chemicals. Additionally, it is used as a disinfectant to kill bacteria and other microorganisms on surfaces and in medical settings.
Question 3: How Long Does It Take for Isopropyl Alcohol to Evaporate?
Answer: The evaporation rate of isopropyl alcohol depends on several factors such as temperature, pressure, and the concentration of the alcohol in the solution. Generally, isopropyl alcohol will evaporate at a rate of about 10-20 minutes, though this rate can vary depending on the conditions.
Question 4: Is Isopropyl Alcohol Dangerous?
Answer: Isopropyl alcohol can be hazardous if not handled properly. It is flammable and should be kept away from heat and open flames. Inhaling isopropyl alcohol vapors can cause irritation to the eyes, nose, and throat. Prolonged or repeated exposure to isopropyl alcohol can cause damage to the respiratory and nervous systems, and can increase the risk of developing cancer.
Question 5: Does Isopropyl Alcohol Damage Surfaces?
Answer: Isopropyl alcohol is a solvent and can damage some surfaces if not used correctly. It can remove protective coatings, damage finishes, and degrade plastics and other materials. When using isopropyl alcohol on surfaces, it is important to test it in an inconspicuous area first to ensure that it won’t cause any damage.
Question 6: What is an Alternative to Isopropyl Alcohol?
Answer: An alternative to isopropyl alcohol is ethyl alcohol, commonly referred to as ethanol. Ethanol is a clear, colorless liquid with a mild odor and a bitter taste. It is typically used as an antiseptic, disinfectant, and solvent. It is often used in cosmetics, perfumes, and pharmaceuticals. Ethanol is less toxic than isopropyl alcohol, and is a safer alternative for some applications.
Evaporation: Isopropyl vs. water
It is clear that isopropyl alcohol evaporates quickly compared to other liquids, with a half-life of around two to three hours. This means that it is a great choice for cleaning or disinfecting surfaces, but it’s important to remember that it is flammable and should be handled with care. With proper safety precautions, isopropyl alcohol is a fast and effective way to clean and disinfect.