Last Updated on 1 year by Francis
Water is one of the essential elements of life and has many unique properties. Its unique V-shape is one of the most fascinating and least understood. So, why is water V-shaped? In this article, we’ll explore the science behind how water molecules form a V-shape, the implications of this phenomenon, and the potential applications of this knowledge. We’ll also discuss the role that temperature, surface tension, and pressure play in shaping the molecules of water. So, let’s dive into the world of water and discover why water is V-shaped.
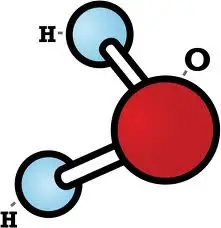
Water molecules have a V-shaped structure due to a phenomenon known as hydrogen bonding. Hydrogen atoms have a positive charge, while oxygen atoms have a negative charge. This creates a dipole, meaning that the molecules of water are attracted to each other. This attraction allows the molecules to bind together in a V-shape, with two hydrogen atoms on either side of the oxygen atom.

Contents
What is the Significance of the V-Shaped Water Molecule?
The water molecule is unique in its molecular structure, as it has a V-shaped molecular geometry. This V-shape is a result of the water molecule’s two hydrogen atoms being held together by a single oxygen atom. The V-shape has a number of implications for the physical and chemical properties of water, making it an important part of our everyday lives.
The V-shape of the water molecule gives it a number of unique properties. The angle of the V-shape helps the water molecule form hydrogen bonds with other molecules, allowing it to interact with other molecules in a variety of ways. This is what gives water its ability to dissolve many substances, as well as its ability to form strong bonds with other molecules. It also allows water molecules to stick together and form surface tension, which is what allows it to form droplets and puddles.
The V-shape of the water molecule also has implications for its electrical properties. The arrangement of the hydrogen atoms creates a dipole, meaning that one side of the molecule is slightly positively charged, while the other side is slightly negatively charged. This gives water molecules a slight electrical charge, which can be used to attract other molecules with opposite charges. This allows water molecules to act as an electrolyte, allowing ions to move freely in solution.
How Does the V-Shape Affect Water’s Reactivity?
The V-shape of the water molecule allows it to interact in a variety of ways with other molecules. The hydrogen bonds formed by the V-shape allow the water molecule to form strong bonds with other molecules, which makes it a very reactive molecule. This means that it can react with other molecules in a variety of ways, allowing it to form new compounds and reactions.
The V-shape of the water molecule also allows it to form hydrogen bonds with other molecules that have a slightly different structure. This helps water molecules to form more stable structures, such as hydrogen-bonded networks, which are important in a variety of biochemical reactions. This allows water to be an important component of many biochemical reactions, as it helps to stabilize the reactants and products.
The Role of the V-Shape in Biological Processes
The V-shape of the water molecule plays an important role in a variety of biological processes. The hydrogen bonds formed by the V-shape help to stabilize proteins and other molecules in cells, which helps to maintain the structure and function of cells. Water molecules also help to transport molecules in and out of cells, as well as to maintain the proper pH balance in cells.
The V-shape also helps to regulate the temperature of cells, as the hydrogen bonds formed by the V-shape are able to absorb and release heat energy. This helps to keep the temperature of cells within a certain range, which is important for the proper functioning of cells.
The Role of the V-Shape in the Environment
The V-shape of the water molecule has a number of implications for the environment. The hydrogen bonds formed by the V-shape help to stabilize molecules in the atmosphere, which helps to prevent them from reacting with other molecules and forming more harmful compounds. The V-shape also helps to regulate the temperature of the atmosphere, as the hydrogen bonds are able to absorb and release heat energy.
The V-shape of the water molecule also helps to form surface tension, which allows water to form droplets and puddles. This helps to regulate the amount of moisture in the atmosphere, as the water droplets are able to absorb and release water vapor. This helps to keep the atmosphere from becoming too dry or too humid, which can have a number of implications for the environment.
The Role of the V-Shape in Chemistry
The V-shape of the water molecule is important in a variety of chemical reactions. The hydrogen bonds formed by the V-shape allow it to interact with other molecules in a variety of ways, allowing it to form new compounds and reactions. This makes it an important component of many chemical reactions, as it helps to stabilize the reactants and products.
The V-shape also helps to regulate the pH of solutions, as the hydrogen bonds formed by the V-shape can absorb and release hydrogen ions. This helps to keep solutions within a certain pH range, which is important for the proper functioning of many chemical reactions.
The Role of the V-Shape in Geology
The V-shape of the water molecule has a number of implications for geology. The hydrogen bonds formed by the V-shape help to stabilize the structure of rocks and other geological formations, as well as to keep them from eroding or being broken down. The V-shape also helps to regulate the temperature of rocks and other geological formations, as the hydrogen bonds are able to absorb and release heat energy.
The V-shape also helps to form surface tension, which allows water to form droplets and puddles. This helps to regulate the amount of moisture in rocks and other geological formations, as the water droplets are able to absorb and release water vapor. This helps to keep rocks and other geological formations from becoming too dry or too humid, which can have a number of implications for the environment.
Top 6 Frequently Asked Questions
What is the scientific explanation for why water is V shaped?
The scientific explanation for why water is V shaped is due to surface tension. Surface tension is a physical property of a liquid, which results from the cohesive bonding between molecules of the same liquid, allowing it to form a thin film on the surface. The molecules at the surface of the liquid have fewer adjacent molecules than those inside the liquid, creating an imbalance of forces, known as surface tension. This causes the liquid to form a V shape when it is poured from a container.
How does surface tension affect the shape of water?
Surface tension affects the shape of water by creating an imbalance of forces. As water is poured from a container, it forms a V shape due to the molecules at the surface of the liquid having fewer adjacent molecules than those inside the liquid. This creates a tension in the surface molecules, which causes them to pull together and form a V shape.
What other liquids form a V shape when poured?
Other liquids that form a V shape when poured include alcohol, glycerol, mercury, and some oils. These liquids also have surface tension, which creates an imbalance of forces and causes them to form a V shape when they are poured from a container.
How is the V shape of water used in everyday life?
The V shape of water is used in everyday life in a variety of ways. For example, when washing dishes, the V shape of the water helps to break up grease and dirt particles, making them easier to wash away. The V shape of water also helps to hold soap bubbles together, which helps to create a lather. Additionally, the V shape of water is used in various scientific experiments and applications, such as measuring the surface tension of a liquid.
Are there any other physical properties of water that affect its shape?
Apart from surface tension, there are several other physical properties of water that affect its shape. These include its viscosity, density, and evaporation rate. The viscosity of water affects the rate of flow, which can make it form a different shape when poured. The density of water affects its buoyancy, which can also affect its shape when poured. The evaporation rate of water can make it form a different shape when exposed to heat or air.
Is the V shape of water unique to liquid state?
No, the V shape of water is not unique to the liquid state. When water is frozen, it still forms a V shape due to the forces of surface tension. The molecules at the surface of the frozen water have fewer adjacent molecules than those inside, creating an imbalance of forces that causes the water to form a V shape.
Why is water polar? Why does water have a bent shape?
Water is an essential part of life on our planet. It shapes the environment we live in and is essential for the survival of all living things. This is why it is important to understand why water is V-shaped. By understanding the structure of water molecules, we can better understand how water behaves and how it affects the environment around us. By understanding the importance of water, we can ensure that we are taking the necessary steps to conserve it and preserve it for future generations.

.jpg)
.jpg)