Last Updated on 1 year by Francis
Ions are a vital part of our world, yet many of us may not know where to find them or even what they are. But fear not, here you will learn all about ions and where they can be found. From the air we breathe to the water we drink, ions are all around us, and understanding them can help us to appreciate the intricate workings of our environment. So, let’s dive in and discover where exactly ions can be found.
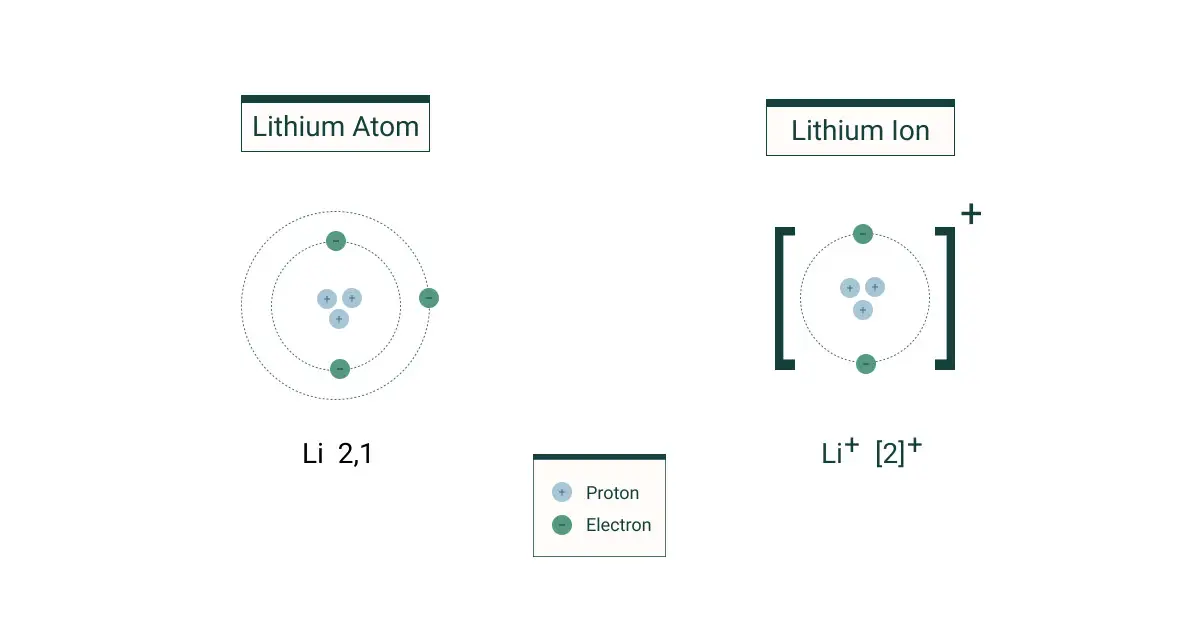
Ions are found in a variety of places, including the atmosphere, oceans, and within all living things. They are also found in rocks, minerals and soil. Ions are formed when atoms or molecules gain or lose electrons, becoming charged particles. Positively charged ions, or cations, are produced when atoms or molecules lose electrons, while negatively charged ions, or anions, are produced when atoms or molecules gain electrons.

Contents
Where Are Ions Located?
Ions are atoms or molecules that have been charged by either gaining or losing electrons. These charged particles can be found in a variety of places, from the air we breathe to the food we eat. In this article, we will explore the different locations where ions can be found.
In the Atmosphere
Ions are naturally found in the atmosphere. They are created by the interaction of cosmic rays with the molecules in the air, creating an ionized form of the molecules. These ions can then interact with other molecules in the atmosphere, resulting in a continual cycle of ion creation and destruction. The amount of ions present in the atmosphere can vary depending on the time of day, the season, and the altitude.
Ions can also be found in the atmosphere as a result of human activities such as burning fossil fuels, which releases particles that can be charged. These particles can then interact with other molecules in the atmosphere, creating ions.
In Water
Ions are also present in water. When water is dissociated, it produces positively and negatively charged particles called ions. These ions can then interact with other molecules in the water, resulting in a variety of chemical reactions. The presence of ions in water can affect the taste, odor, and even the color of the water.
In addition, ions can be found in water as a result of human activities such as pollution. Pollution can introduce a variety of different ions into the water, which can have a negative impact on the environment.
In Living Organisms
Ions can also be found in living organisms. These ions are necessary for a variety of metabolic processes, such as maintaining the balance of electrolytes in the body. Ions are also involved in the transmission of nerve signals, as well as the regulation of cell membrane potential.
Ions can also be found in food and drink. Many foods and beverages contain minerals that can be charged, resulting in the presence of ions. For example, salt is an ionic compound that is composed of positively charged sodium ions and negatively charged chlorine ions.
In Manufacturing
Ions are also used in a variety of manufacturing processes. For example, ions can be used to create an electrical charge, which can then be used to create a variety of electrical components. In addition, ions can be used to clean surfaces, as well as to create coatings and protective layers.
Ions can also be used in chemical processes. For example, ions can be used to catalyze chemical reactions, as well as to purify and separate compounds. Ions can also be used to create desired compounds from raw materials.
In Medicine
Ions are also used in a variety of medical applications. For example, ions can be used to diagnose and treat various diseases. Ions can also be used to create drugs and pharmaceuticals, as well as to deliver therapeutic treatments.
In addition, ions can be used to detect and analyze biological samples. For example, ions can be used to detect the presence of certain molecules or compounds in a sample, as well as to measure their concentrations.
In Electronics
Ions can also be found in a variety of electronic devices. For example, ions can be used to create electrical circuits, as well as to store and transfer electrical charges. In addition, ions can be used to create displays, as well as to control the flow of electricity.
Ions can also be used to create sensors and transducers. For example, ions can be used to create pressure, temperature, and humidity sensors, as well as to create sensors for detecting light and sound.
In Other Applications
Ions can also be found in a variety of other applications. For example, ions can be used to create paints and coatings, as well as to create fertilizers and soil enhancers. In addition, ions can be used to create adhesives and sealants, as well as to create a variety of other products.
Related Faq
What are Ions?
Ions are atoms or molecules that carry an electric charge due to the loss or gain of electrons. They can be positively charged (known as cations) or negatively charged (known as anions). Ions play an important role in many chemical processes such as the formation of salts and electrolysis.
Where are Ions Found?
Ions can be found in a variety of places, including in nature, in laboratories, and in industrial processes. In nature, ions can be found in the atmosphere, in rivers and streams, and in the ocean. In laboratories and industrial processes, ions are used to generate electricity, in chemical synthesis and analysis, and in the production of pharmaceuticals.
How are Ions Formed?
Ions are formed when atoms or molecules gain or lose electrons. This process is known as ionization and can occur through a variety of means, including exposure to ultraviolet radiation, chemical reactions, and electrical currents. For example, when an atom or molecule is exposed to ultraviolet light, electrons are knocked off of the atom, creating a positively charged ion.
What is the Difference Between Anions and Cations?
Anions are negatively charged ions and cations are positively charged ions. Anions are formed when an atom or molecule gains electrons and cations are formed when an atom or molecule loses electrons. An example of an anion is Cl-, which is formed when chlorine gains an electron, and an example of a cation is Na+, which is formed when sodium loses an electron.
What is the Role of Ions in Chemical Reactions?
Ions play an important role in many chemical reactions. They are necessary for the formation of salts and for the transfer of electrons in oxidation-reduction reactions. Additionally, ions can be used to catalyze reactions, allowing them to occur more quickly. In many cases, ions are necessary for the formation of specific bonds between atoms, as well as for the formation of molecules.
Are There Different Types of Ions?
Yes, there are different types of ions. Some of the most common types of ions include monatomic ions, polyatomic ions, and complex ions. Monatomic ions are formed when a single atom gains or loses electrons, polyatomic ions are formed when two or more atoms bond together and gain or lose electrons, and complex ions are formed when two or more atoms bond together and form an ionic bond.
Finding Charges of Ions on Periodic Table
In conclusion, ions are found in many places, from natural bodies of water to the air we breathe. They are essential elements in numerous chemical reactions and are essential for life to exist. While they can be found in many places, the presence of ions in the atmosphere and water is particularly important for the health of the planet. Therefore, it is essential that we understand where ions are found and the role they play in our environment.