Last Updated on 1 year by Francis
Water is one of the most essential elements of life, but have you ever stopped to ask yourself: what is it made of? On the surface, water may seem like a simple substance, but its composition is surprisingly complex. In this article, we’ll take a closer look at the science behind water and explore what it’s made of.
Contents
What is Water Composed of?
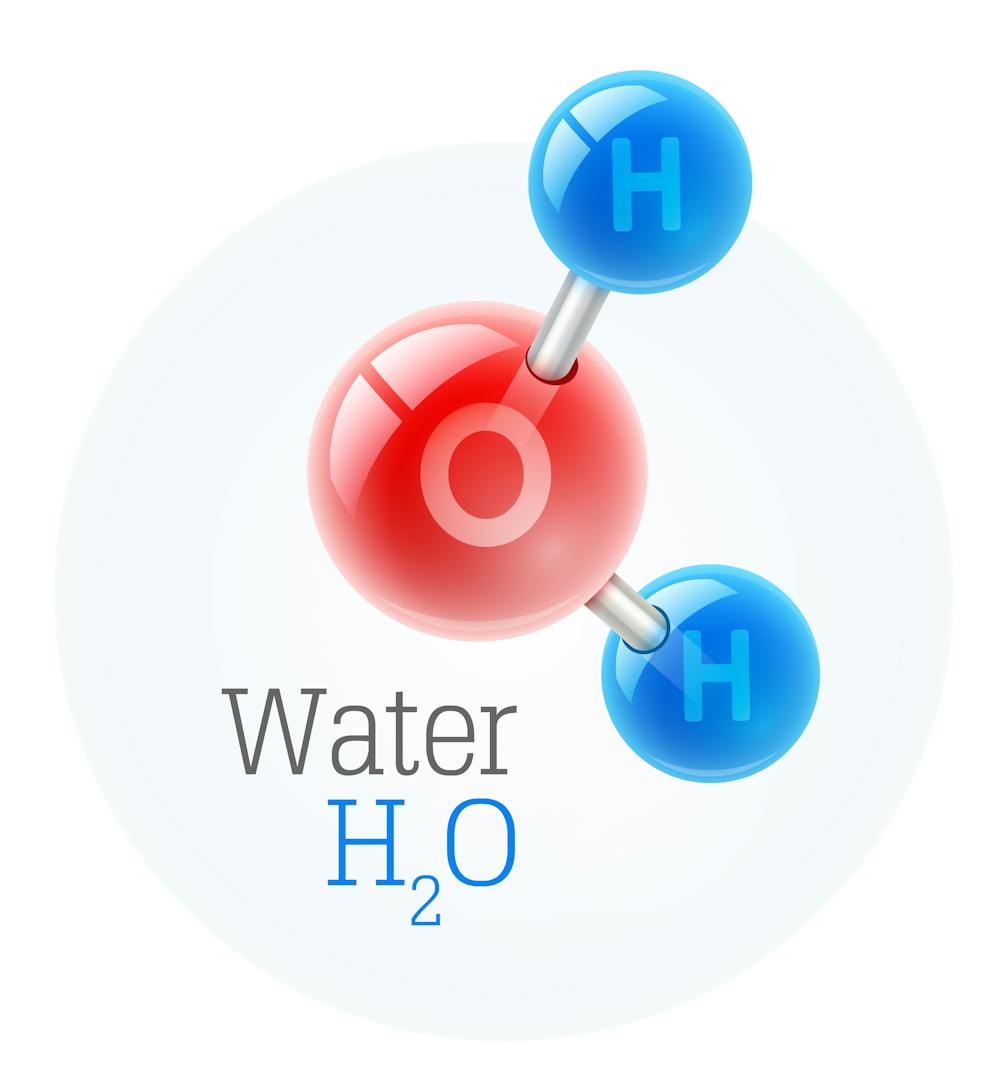
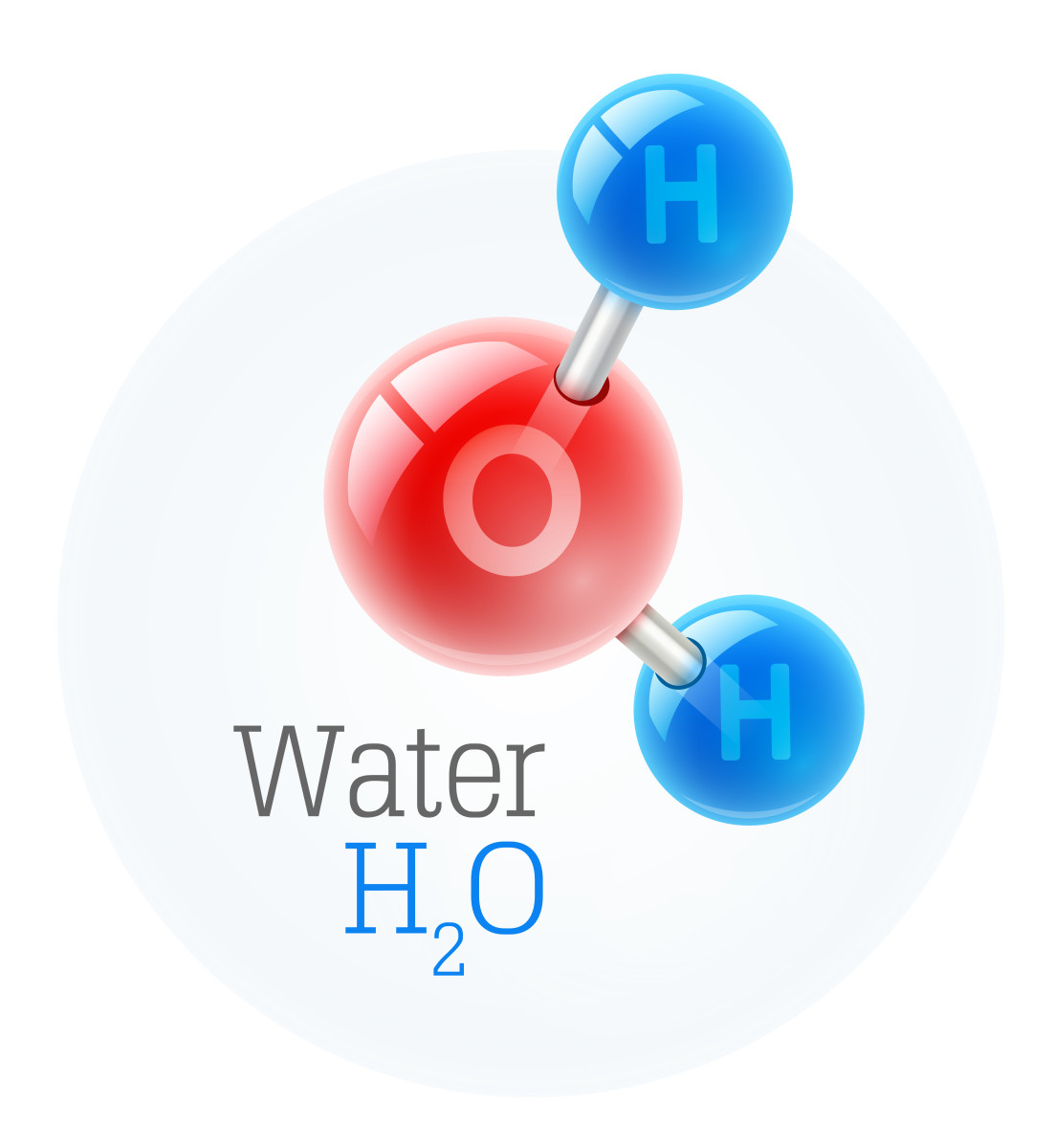
Water is an essential component of all life on Earth, and is composed of two elements: hydrogen and oxygen. These two elements make up the majority of water, but there are other elements present in small amounts. Water is a very simple molecule, but it is incredibly important for the survival of all life on Earth.
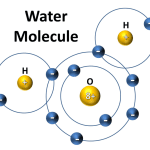
Water is composed of two hydrogen atoms and one oxygen atom. This combination creates a molecule (H2O) that is very stable and can exist in all three states of matter: solid, liquid, and gas. The hydrogen atoms are slightly positively charged, while the oxygen atom is slightly negatively charged. This allows water molecules to be attracted to each other, forming hydrogen bonds. This is what gives water its unique properties, such as its ability to dissolve many different substances and its high surface tension.
Water is also made up of other elements in trace amounts, such as carbon, nitrogen, sulfur, and phosphorus. These elements are essential for life, as they are necessary for the formation of proteins and other molecules. The exact composition of water varies depending on its source, but the two main elements (hydrogen and oxygen) remain the same.
The Properties of Water
Water is a very unique molecule due to its special properties. Its ability to dissolve many different substances is what makes it so vital for life. It is also able to absorb heat and release it slowly, which helps to regulate temperatures on Earth. Water also has a high surface tension, which allows it to form droplets and hold its shape.
The hydrogen bonds between water molecules also play an important role. These bonds have a greater strength than other intermolecular forces, which gives water an unusually high boiling point and freezing point. This is why water can exist in all three states of matter, and why it is able to support life.
The Importance of Water
Water is essential for all life on Earth. It is the main component of cells and is used to transport nutrients and other molecules throughout the body. It is also necessary for the chemical reactions that occur in the body, and helps to regulate temperatures.
Water is also used in many industries, such as agriculture, manufacturing, and energy production. It is used to clean and sterilize surfaces, and to transport materials and products. In addition, it is used to generate electricity and to provide drinking water to millions of people around the world.
Conclusion
Water is an essential component of all life on Earth, and is composed of two main elements: hydrogen and oxygen. These two elements make up the majority of water, but there are other elements present in small amounts. Water has many unique properties, such as its ability to dissolve many different substances and its high surface tension. These properties make it vital for life, and it is also used in many industries for a variety of purposes.
Related Faq
What is Water Made of?
Answer: Water is composed of two hydrogen atoms and one oxygen atom. It is a compound made of two parts hydrogen and one part oxygen, with a chemical formula of H2O. Water is an essential element for life and is the most abundant compound found in the Earth’s biosphere. It is also the only compound found naturally in all three states of matter: solid, liquid and gas.
What do Hydrogen and Oxygen Do in Water?
Answer: Hydrogen and oxygen are two of the most common elements found in the universe. In water, hydrogen and oxygen combine to form hydrogen bonds, which form the structural backbone of water molecules. Hydrogen bonds are very strong and are responsible for many of the properties that make water such an important substance for life on Earth. For example, hydrogen bonds create surface tension, which allows water to form droplets and to be able to travel up narrow tubes. Hydrogen bonds also give water an unusually high boiling point and enable it to dissolve a wide range of substances.
What are the Properties of Water?
Answer: Water has many unique physical and chemical properties, some of which make it essential for life. Water has a high boiling point and freezing point, which makes it a liquid at a wide range of temperatures. Water is also highly cohesive and adhesive, meaning it sticks to itself and other substances. This enables it to form droplets, travel up narrow tubes, and form bridges between molecules. Water is also an excellent solvent and is able to dissolve many different substances. Additionally, water has a high specific heat, meaning it takes a lot of energy to heat it up or cool it down.
What Does Water Do for the Human Body?
Answer: Water is essential for life and is vital for the human body. Water makes up about 60% of an adult’s body weight and is involved in virtually all of the body’s processes. Water helps to transport nutrients and oxygen around the body, regulate body temperature, and remove waste and toxins. It also helps to lubricate joints, cushion organs, and maintain a healthy balance of electrolytes. Not drinking enough water can cause dehydration, which can lead to a range of health problems.
What are the Different Forms of Water?
Answer: Water can exist in all three states of matter: solid, liquid, and gas. In its solid state, water is known as ice, and in its gaseous state, it is known as water vapor. Water vapor is an important component of the atmosphere, and it is responsible for the formation of clouds. Water can also exist in liquid form, which is the most common form of water and is the form that is essential for life.
What is the Chemical Formula of Water?
Answer: The chemical formula of water is H2O, which stands for two hydrogen atoms and one oxygen atom. Water is a polar molecule, meaning it has a slightly positive charge on one side and a slightly negative charge on the other. This enables it to form hydrogen bonds, which are responsible for many of the properties of water.
What is water made of?
Water is an essential part of life, and its composition is incredibly important. Through this article, we have seen that water is made up of two hydrogen atoms and one oxygen atom. This unique combination of elements is what makes water so essential for life on Earth, and it is why we must take steps to conserve and protect our water resources. With the right understanding and care, we can ensure that we have clean, safe water for generations to come.



.jpg)


