Last Updated on 1 year by Francis
Salt is an essential ingredient in our daily diet, and it’s also an important factor in maintaining our bodily functions. But, does it really have a positive ionic charge? In this article, we will explore the science behind salt and its ionic charge, and see if it really is a positive ion. So, let us find out if salt is a positive ion and how it affects us and our environment.
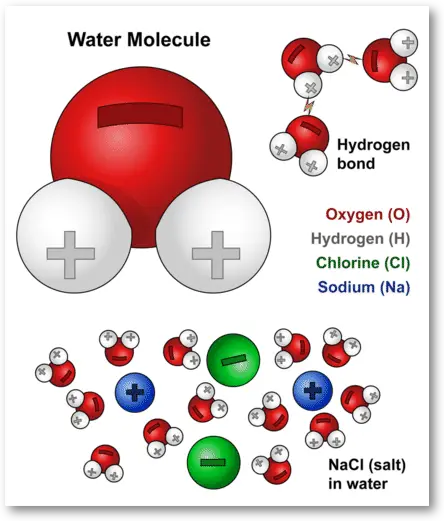
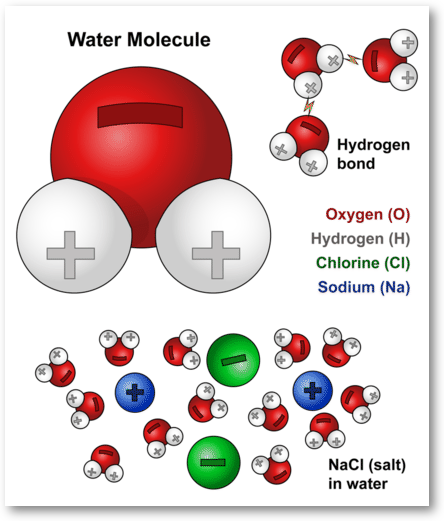
Salt is a neutral ion, not a positive ion. Salt is an ionic compound made up of two ions: a positively charged ion called a cation and a negatively charged ion called an anion. The cation in salt is sodium and the anion is chlorine. Together, they form a neutral compound.
Contents
Is Salt a Positively Charged Ion?
Salt, or sodium chloride, is a compound that consists of two elements, sodium and chlorine. Both of these elements form ions, which are atoms that are electrically charged. Sodium has a single positive charge, while chlorine has a single negative charge. This means that when combined, the two ions create a neutral salt molecule. However, when the salt is dissolved in water, the ions separate and become charged, with the sodium ions having a positive charge and the chloride ions having a negative charge.
Salt is composed of two different ions, sodium and chloride. Sodium is a positively charged ion, while chloride is a negatively charged ion. When these two ions combine, they form a neutral salt molecule. This means that in the solid form, the salt has no charge. However, when the salt is dissolved in water, the ions separate and become charged. The sodium ions have a positive charge, while the chloride ions have a negative charge. This means that when salt is dissolved in water, it will have a net positive charge.
The positive charge of the sodium ions is due to the fact that sodium has one more proton than electron. This creates an imbalance of positive and negative charges, resulting in a net positive charge. The chloride ions, on the other hand, have an equal number of protons and electrons. This creates a balanced charge, resulting in a negative charge.
What Does a Positively Charged Salt Mean?
A positively charged salt means that when the salt is dissolved in water, it will have a net positive charge. This is due to the fact that the sodium ions have a positive charge, while the chloride ions have a negative charge. This means that when salt is dissolved in water, it will attract positively charged particles, such as protons, and repel negatively charged particles, such as electrons.
The positive charge of the salt can have a number of effects on the surrounding environment. For example, it can be used to attract positively charged particles, such as protons, and repel negatively charged particles, such as electrons. This can be useful in a variety of applications, such as in the production of batteries and in water purification systems.
What Are the Effects of Positively Charged Salt?
The positive charge of the salt can have a number of effects on the surrounding environment. For example, it can be used to attract positively charged particles, such as protons, and repel negatively charged particles, such as electrons. This can be useful in a variety of applications, such as in the production of batteries and in water purification systems.
In addition, the positively charged salt can act as an electrolyte, which means it can conduct electricity. This can be beneficial in a variety of applications, such as in the production of electronics and in the medical field. The positively charged salt can also be used to create a buffer, which can be beneficial in the manufacturing process.
Conclusion
In conclusion, salt is a compound that consists of two elements, sodium and chlorine. When the salt is dissolved in water, the ions separate and become charged, with the sodium ions having a positive charge and the chloride ions having a negative charge. This means that when salt is dissolved in water, it will have a net positive charge. The positive charge of the salt can have a number of effects on the surrounding environment, such as being used to attract positively charged particles and repel negatively charged particles. In addition, the positively charged salt can act as an electrolyte and can be used to create a buffer.
Related Faq
What is a Positive Ion?
A positive ion is an atom or molecule that has lost one or more electrons, leaving it with a net positive charge. Positive ions consist of atoms that have either lost one or more electrons, or atoms that have gained protons.
Is Salt a Positive Ion?
No, salt is not a positive ion. Salt, or sodium chloride, is a neutral molecule composed of positively-charged sodium ions and negatively-charged chloride ions. The positive and negative charges balance each other, making salt a neutral molecule.
What is the Chemical Formula for Salt?
The chemical formula for salt is NaCl, which stands for sodium chloride. This indicates that salt is composed of one atom of sodium and one atom of chlorine.
How is Salt Formed?
Salt is formed through the process of electrolysis. This process involves the passage of an electric current through a solution of brine, or a solution of salt and water. As the current passes through the solution, the salt molecules separate into positively-charged sodium ions and negatively-charged chloride ions.
Where is Salt Found?
Salt is found naturally in many places in the world. It is often found in salt deposits left behind by ancient oceans, as well as in underground deposits created by the evaporation of salty lakes. Salt is also found in many of the world’s oceans, seas, and other bodies of water.
What is Salt Used For?
Salt is used for a variety of purposes, both in the kitchen and in industrial applications. In the kitchen, salt is used to season food, as well as to preserve it. In industrial applications, salt is used in the production of chlorine, sodium hydroxide, and other chemicals. It is also used for de-icing roads and sidewalks in the winter.
Do Salt Lamps Work?
In conclusion, salt is both a positive and negative ion, depending on its chemical structure. Salt is composed of two elements, sodium and chlorine, and these elements can exist in either positive or negative forms. The positive ions of salt are responsible for its taste, while the negative ions are responsible for its ability to dissolve in water. Salt is a common ingredient in many dishes and it can be used to season food, as well as to preserve food. Therefore, it is safe to say that salt is a positive ion.