Last Updated on 1 year by Francis
Oxygen, the most abundant element in our universe, is a vital component of all known forms of life. But is it a cation or anion? This intriguing question can be answered by exploring the molecular structure of oxygen and its various forms. In this article, we will explore the answer to this question and why it matters.
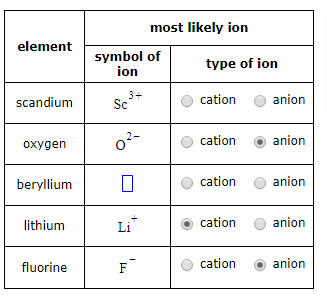
Oxygen is an anion. An anion is an ion that has a negative charge. It is formed when an atom gains electrons. Oxygen is the most abundant element in the Earth’s crust, making up around 46% of the Earth’s crust by mass.
An anion is the opposite of a cation, which is an ion with a positive charge. Cations are formed when an atom loses electrons.
Contents
What is an Anion and a Cation?
Anions and cations are both ions, or atoms with an electrical charge. An anion is a negatively charged ion, while a cation is a positively charged ion. Ions are formed when an atom gains or loses electrons, resulting in the atom having an electrical charge. The electrical charge of an ion determines whether it is an anion or a cation.
Anions are atoms that have gained electrons, resulting in a negatively charged atom. Cations are atoms that have lost electrons, resulting in a positively charged atom. Both anions and cations are attracted to oppositely charged ions due to electrostatic forces, resulting in the formation of ionic compounds.
What is Oxygen?
Oxygen is a chemical element with the symbol O and atomic number 8. It is a nonmetal and is the most abundant element in Earth’s atmosphere, making up about 21% of the atmosphere by volume. Oxygen is a gas at normal temperature and pressure and is colorless, odorless, tasteless, and non-toxic.
Oxygen is important for all aerobic life forms, as it is necessary for cellular respiration. It is also used in a variety of industrial processes, such as welding, combustion of fuels, and purification of water. Oxygen is also used in rocket propulsion and in the production of steel.
Is Oxygen an Anion or a Cation?
Oxygen is not a cation or an anion. It has an atomic number of 8, which means it has 8 electrons in its neutral state. Oxygen is a nonmetal and does not typically gain or lose electrons. Therefore, oxygen does not form ions, and therefore it is neither an anion nor a cation.
What are Oxyanions?
Oxyanions are ions that contain oxygen and another element. These ions are formed when oxygen atoms gain or lose electrons, resulting in the formation of an ion with an electrical charge. Oxyanions have the same electrostatic properties as anions and cations, and they can form ionic compounds with oppositely charged ions.
What are Examples of Oxyanions?
There are many examples of oxyanions, including nitrate (NO3-), sulfate (SO4-2), and phosphate (PO4-3). These oxyanions are formed when oxygen atoms gain electrons, resulting in a negatively charged ion.
What is the Difference Between Oxyanions and Anions?
The main difference between oxyanions and anions is that oxyanions contain oxygen atoms, while anions may contain any other element. Anions are formed when atoms gain electrons, resulting in a negatively charged ion. Oxyanions are formed when oxygen atoms gain electrons, resulting in a negatively charged ion.
Do Oxyanions Behave Like Anions or Cations?
Oxyanions behave like anions and can form ionic compounds with oppositely charged ions. Oxyanions are attracted to positively charged ions due to electrostatic forces, resulting in the formation of ionic compounds.
What are Examples of Oxyanions Forming Ionic Compounds?
Examples of oxyanions forming ionic compounds include sodium nitrate (NaNO3), ammonium sulfate (NH4+SO4-2), and magnesium phosphate (MgPO4). In these examples, the oxyanions are attracted to the oppositely charged cations, resulting in the formation of ionic compounds.
Do Oxyanions Exist in Nature?
Oxyanions are a common form of ionic compounds in nature. In the atmosphere, oxyanions such as nitrate, sulfate, and phosphate are important components of air pollution and can have negative effects on human health. In the ocean, oxyanions are important components of marine life, and they can have positive effects on marine ecosystems.
Top 6 Frequently Asked Questions
Question 1: Is Oxygen a Cation or Anion?
Answer: Oxygen is an anion. An anion is an atom or molecule that has a net negative charge due to the presence of more electrons than protons. Oxygen is a very common anion, as it is a component of many compounds found in nature, such as water. In its elemental form, oxygen has 8 protons and 8 electrons, giving it a net negative charge. Oxygen anions are important in many biological processes, including the formation of proteins and the transport of oxygen in the bloodstream.
Question 2: What is the Charge of an Oxygen Anion?
Answer: The charge of an oxygen anion is -2. This is due to the fact that oxygen has 8 protons and 8 electrons, giving it a net negative charge. Oxygen is the most common anion found in nature, and is important in many biological processes, including the formation of proteins and the transport of oxygen in the bloodstream.
Question 3: What are the Properties of Oxygen Anions?
Answer: Oxygen anions have a number of properties, including a net negative charge (-2), a high electronegativity, and a high affinity for electrons. Oxygen anions are also highly reactive, meaning they will readily form bonds with other atoms or molecules. This makes oxygen anions a key component of many compounds found in nature, such as water.
Question 4: What is the Chemical Formula for Oxygen Anions?
Answer: The chemical formula for oxygen anions is O2- . This formula reflects the fact that oxygen has 8 protons and 8 electrons, giving it a net negative charge. As an anion, oxygen is highly reactive and forms bonds easily with other atoms or molecules, making it a key component of many compounds found in nature, such as water.
Question 5: What is the Electronegativity of Oxygen Anions?
Answer: The electronegativity of oxygen anions is 3.44. This is due to the fact that oxygen has 8 protons and 8 electrons, giving it a net negative charge. Oxygen anions have a high electronegativity, meaning that they are highly reactive and will readily form bonds with other atoms or molecules. This makes oxygen anions a key component of many compounds found in nature, such as water.
Question 6: What is the Bonding Capacity of Oxygen Anions?
Answer: The bonding capacity of oxygen anions is 2. This is due to the fact that oxygen has 8 protons and 8 electrons, giving it a net negative charge. As an anion, oxygen is highly reactive and forms bonds easily with other atoms or molecules, making it a key component of many compounds found in nature, such as water. Oxygen anions can form two covalent bonds with other atoms or molecules, allowing them to form a wide variety of compounds.
Cations and Anions Explained
In conclusion, Oxygen is neither a cation nor an anion. Oxygen is a neutral atom that can form covalent and ionic bonds, depending on the situation. The influence of other atoms in the compound can cause Oxygen to either gain or lose electrons, forming either a cation or an anion. Ultimately, Oxygen’s ability to form cations and anions gives it the capability to form a variety of compounds and be a part of a variety of chemical reactions.