Last Updated on 1 year by Francis
Electromotive force, commonly referred to as EMF, plays a crucial role in understanding the behavior of electrical circuits. In chemistry, EMF is defined as the amount of energy that a battery, fuel cell, or other source of electrical energy can produce per unit of charge when positive and negative charges are separated from each other. The EMF formula in chemistry is used to calculate the potential difference between two electrodes and plays a significant role in determining the overall efficiency of a battery or cell. In this context, understanding the EMF formula is essential for anyone dealing with electrical circuits, batteries, and electrochemistry.
Contents
The Basics of Electromotive Force
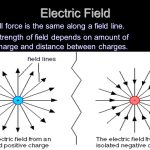
Electromotive force (EMF) is the measure of potential energy in an electrical circuit. It is the “push” that causes electrons to flow through a wire or other electrical conductor. EMF is measured in units of volts (V) and is represented by the symbol “E.”
The Difference Between EMF and Voltage
EMF is often confused with voltage, but the two are not the same. Voltage is the measure of the electrical potential difference between two points in a circuit. EMF, on the other hand, is the total energy supplied by a source (such as a battery or generator) per unit charge. In other words, voltage is the potential difference between two points, while EMF is the total energy supplied by the source.
The EMF Formula
The EMF formula is used to calculate the electromotive force in a circuit. It is given by:
E = V + Ir
Where E is the EMF, V is the voltage, I is the current, and r is the internal resistance of the source.
Understanding the Components of the Formula
The voltage represents the potential difference between the two points in a circuit. The current represents the flow of electrons through the circuit. The internal resistance of the source represents the resistance to the flow of electrons within the source itself.
How to Use the EMF Formula
To use the EMF formula, you need to know the voltage and current in the circuit, as well as the internal resistance of the source. Once you have these values, you can simply plug them into the formula and calculate the EMF.
Applications of EMF
EMF has many applications in the fields of electrical engineering and physics. Some of the most common applications include:
Key takeaway: Electromotive force (EMF) is the measure of potential energy in an electrical circuit, which is different from voltage. EMF can be calculated using the EMF formula, where the internal resistance of the source, voltage, and current in the circuit need to be known. Despite some misconceptions, EMF is not harmful to health, is a natural phenomenon, and has many applications in electrical engineering and physics.
Batteries
Batteries are a common source of EMF. They convert chemical energy into electrical energy, which can then be used to power devices such as flashlights, cell phones, and laptops.
Generators
Generators are another source of EMF. They convert mechanical energy into electrical energy, which can then be used to power homes, businesses, and other electrical devices.
Electromagnets
EMF is also used in the creation of electromagnets. Electromagnets are used in a wide range of applications, from medical imaging to transportation.
Common Misconceptions About EMF
There are many misconceptions about EMF. Some of the most common include:
EMF is Harmful to Your Health
There is no scientific evidence to suggest that EMF is harmful to your health. While high levels of EMF can be dangerous (such as those found near power lines), the levels found in everyday life are typically harmless.
EMF and Radiation are the Same Thing
EMF and radiation are not the same thing. Radiation is a form of energy that can be harmful to your health, such as that emitted by nuclear materials. EMF, on the other hand, is a form of energy that is found in everyday life, such as that produced by electrical devices.
EMF is a New Phenomenon
EMF is not a new phenomenon. It has been present in the environment since the beginning of time, and is a natural part of the world we live in.
Example Calculation
Let’s say you have a battery with a voltage of 12V, and an internal resistance of 0.5 ohms. The circuit has a current of 2A. Using the EMF formula, we can calculate the EMF as follows:
E = 12V + (2A * 0.5 ohms)
E = 12V + 1V
E = 13V
Therefore, the EMF of the circuit is 13V.
FAQs for EMF Formula Chemistry
What is EMF (Electromotive Force) in chemistry?
In chemistry, EMF (Electromotive Force) is defined as the potential difference between two half-cells in an electrochemical cell. It is also called voltage or cell potential. It is a measure of the driving force behind the flow of electrons in a circuit. EMF is usually measured in volts (V) or millivolts (mV).
How do you calculate EMF?
EMF can be calculated using the Nernst equation or the Gibbs-Helmholtz equation. The Nernst equation takes into account the concentration of the reactants and products in the half-cells, as well as the temperature and the charge of the ions. The Gibbs-Helmholtz equation takes into account the change in free energy of the system, which is related to the enthalpy and entropy changes.
What is the Nernst equation?
The Nernst equation relates the equilibrium potential of an electrochemical reaction to the concentration of the reacting species. It is used to calculate the cell potential under non-standard conditions, when the concentration of the species in the half-cells is not 1 M. The Nernst equation is given by: E = E0 – (RT/nF)ln(Q), where E is the cell potential, E0 is the standard potential, R is the gas constant, T is the temperature, n is the number of electrons transferred, F is the Faraday constant, and Q is the reaction quotient.
What is the Gibbs-Helmholtz equation?
The Gibbs-Helmholtz equation relates the change in free energy of a system to the temperature and the change in enthalpy or entropy. It is used to calculate the cell potential under non-standard conditions, when the temperature or the pressure is different from standard conditions. The Gibbs-Helmholtz equation is given by: ΔG = ΔH – TΔS, where ΔG is the change in free energy, ΔH is the change in enthalpy, ΔS is the change in entropy, and T is the absolute temperature.
What is the difference between EMF and potential difference?
EMF (Electromotive Force) is the potential difference between two half-cells in an electrochemical cell, while potential difference is the difference in potential (voltage) between two points in an electrical circuit. EMF is a measure of the driving force behind the flow of electrons, while potential difference is a measure of the work done per unit charge.
What is the unit of EMF?
The unit of EMF (Electromotive Force) is volt (V) or millivolt (mV). It is a measure of the potential difference between two half-cells in an electrochemical cell.
How is EMF used in electrochemical cells?
EMF is used to determine the direction and the magnitude of the electron flow in an electrochemical cell. It is also used to calculate the cell potential under different conditions, such as temperature, pressure, and concentration of the reactants and products. The EMF of a cell can also be used to determine the thermodynamics of a reaction, such as the change in free energy, enthalpy, and entropy.