Last Updated on 1 year by Francis
It’s a common question to ask: are protons positive or negative? In this article, we’ll explore what protons are, how they are classified, and how they interact with other particles. We’ll also look at some examples of how protons are used in everyday life. By the end of this article, you’ll have a better understanding of protons and how they play a role in our universe.
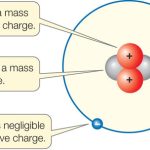
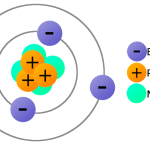
Protons are positively charged particles found in the nucleus of an atom. They are made up of two up quarks and one down quark and have a charge of +1. Protons have a mass of 1.6726 x 10-27 kg and are about 1,836 times more massive than electrons. Protons are not affected by electric and magnetic fields and form the nucleus of an atom along with neutrons.

Contents
What is a Proton and What are Its Charges?
A proton is a subatomic particle with a positive electric charge. It is found in the nucleus of every atom and is one of the main components of all matter. Protons have a mass of 1.6726 x 10^-27 kilograms and are part of the family of particles known as baryons. Protons, along with neutrons, form the nucleus of an atom and are held together by the strong nuclear force.
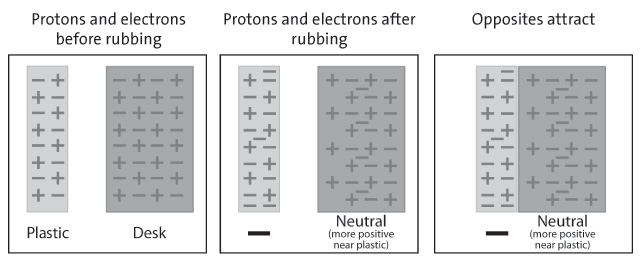
The charge of a proton is +1, which means it is positively charged. This is because it contains one more unit of positive charge than an electron has negative charge. This means that protons and electrons attract each other and form a bond, which is the basis of chemical reactions and is responsible for the different types of matter that we see around us.
How Do Protons Affect Their Surroundings?
The charge of a proton affects its surroundings in a number of ways. For example, the charge of a proton can attract or repel other charged particles. This is why protons are important in the formation of molecules and chemical reactions. Protons also act as an anchor for electrons and other particles, allowing them to move around the nucleus of an atom.
The charge of a proton also affects its ability to absorb and release energy. Protons can absorb and release energy in the form of radiation, which is why they are involved in nuclear reactions. Additionally, the charge of a proton can affect its magnetic properties, as it can be either attracted or repelled by a magnetic field.
What Are the Other Types of Charges?
In addition to protons, there are two other types of charges: electrons and neutrons. Electrons have a negative charge and neutrons have no charge. Electrons are the main component of atoms, as they are responsible for their chemical properties. Neutrons are found in the nucleus of an atom and are responsible for its stability.
What Are the Properties of Protons?
Protons have a number of properties that make them unique. For one, they are extremely stable and have a very long lifetime. This means that they can remain in the same state for a very long time, making them ideal for nuclear reactions. Additionally, protons have a strong magnetic moment, which makes them valuable for use in medical imaging.
What Are the Uses of Protons?
Protons have a number of uses in the scientific and medical fields. For example, they can be used to create medical images, as they are attracted to magnetic fields. Additionally, they are used in nuclear reactors to produce heat and power. Additionally, they can be used to create reactions that can be used in the manufacturing of materials such as plastics and pharmaceuticals.
Conclusion
In conclusion, protons are subatomic particles with a positive electric charge. They are found in the nucleus of every atom and are the main components of all matter. Protons have a number of properties that make them unique, including their strong magnetic moment and their long lifetime. They can be used for a variety of purposes, such as medical imaging and nuclear reactions.
Frequently Asked Questions
Are Protons Positive or Negative?
Answer: Protons are positively charged particles.
Why Are Protons Positively Charged?
Answer: Protons have a positive charge because they contain quarks with a positive charge. Quarks are a type of subatomic particle, and they have a charge of +2/3, which gives the proton a total charge of +1.
What Are Protons Made Of?
Answer: Protons are made up of three quarks. Two of these quarks are up-quarks, which have a charge of +2/3, and one is a down-quark, which has a charge of -1/3. The combination of these three quarks gives the proton a total charge of +1.
What Are the Properties of Protons?
Answer: Protons have a mass of approximately 1.67 x 10^-27 kilograms, and they have a radius of approximately 0.875 femtometers (fm). Protons also have a positive charge of +1, and they are found in the nucleus of an atom.
Where Can Protons Be Found?
Answer: Protons are found in the nucleus of every atom. They are the most abundant type of particle in the nucleus, accounting for 99.9% of the mass inside the nucleus. Protons are also found in the form of free protons, which are not bound to a nucleus.
What Role Do Protons Play in Chemistry?
Answer: Protons play an important role in chemistry, as they determine the properties of an atom. The number of protons in an atom determines the element that it is, and this in turn determines its chemical properties. Protons also interact with other particles, such as electrons and neutrons, to form molecules and compounds.
Why Is An Electron Negative In Charge
In conclusion, it is clear that protons are positively charged particles, as opposed to electrons which are negatively charged. This is due to protons having an abundance of positive charge, while electrons have an equal amount of negative charge. This understanding of protons and electrons is essential to understanding basic atomic structure, and it is important to remember that protons are positively charged particles.