Last Updated on 1 year by Francis
As a society, we rely heavily on batteries to power our daily lives. However, are batteries as harmless as we think? A growing body of research suggests that batteries may be negatively charged, both environmentally and personally, in ways that we may not be aware of. In this article, we’ll explore the potential consequences of using batteries and how we can reduce their impact.
language
Contents
What Exactly Is a Battery?
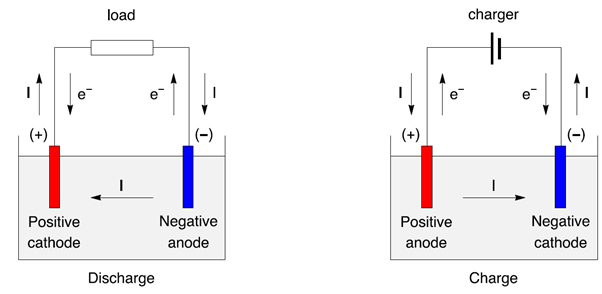
A battery is an electrochemical device that converts chemical energy into electrical energy. It is made up of one or more electrochemical cells, each of which contains two electrodes (an anode and a cathode) immersed in an electrolyte. When the electrodes are connected to an external circuit, the chemical energy is converted into electrical energy. The most common type of battery is a lead-acid battery, which is commonly used in cars and other motorized vehicles.
Batteries are used to store electrical energy and can be recharged by applying an external voltage. This process is known as charging and the battery is said to be “positively charged” when the external voltage is greater than the battery’s voltage. Conversely, the battery is said to be “negatively charged” when the external voltage is lower than the battery’s voltage.
Are Batteries Negatively Charged?
The simple answer is no, batteries are not negatively charged. Batteries only generate positive electricity; they do not generate negative electricity. When a battery is connected to an external circuit, the electrons flow from the negative terminal to the positive terminal, generating a positive charge.
However, when a battery is being discharged (i.e. used to power a device), the electrons flow from the positive terminal to the negative terminal, resulting in a negative charge. This is why a battery will gradually become weaker as it is discharged.
The Difference Between Positive and Negative Charges
The difference between positive and negative charges is that positive charges have a higher potential energy than negative charges. This means that when a battery is connected to an external circuit, the electrons will flow from the negative terminal to the positive terminal, generating a positive charge. Conversely, when a battery is being discharged, the electrons will flow from the positive terminal to the negative terminal, resulting in a negative charge.
The difference between positive and negative charges can be better understood by looking at the atomic structure of a battery. A battery consists of two electrodes (an anode and a cathode) that are separated by an electrolyte. When a battery is connected to an external circuit, the electrons will flow from the anode to the cathode, generating a positive charge. Conversely, when a battery is being discharged, the electrons will flow from the cathode to the anode, resulting in a negative charge.
What Are Negative Charges Used For?
Negative charges are used for a variety of purposes. For example, they are used in electric motors and generators to create a magnetic field. They are also used in batteries to create a potential difference and allow for the flow of electrons. Finally, negative charges are also used in semiconductors to create a flow of current.
How Are Batteries Charged?
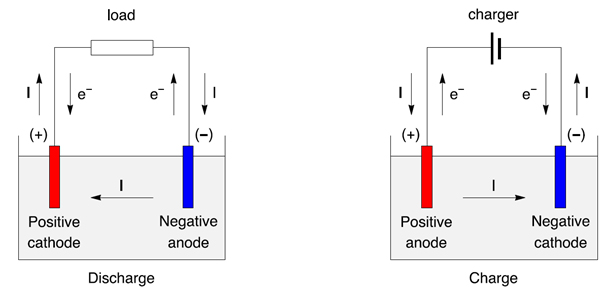
Batteries are typically charged by applying an external voltage. This process is known as charging and the battery is said to be “positively charged” when the external voltage is greater than the battery’s voltage. Conversely, the battery is said to be “negatively charged” when the external voltage is lower than the battery’s voltage.
The most common type of battery charger is a lead-acid battery charger, which is used in cars and other motorized vehicles. This type of charger works by applying an external voltage to the battery, which causes the electrons to flow from the negative terminal to the positive terminal, generating a positive charge.
What Is an Overcharge?
An overcharge occurs when a battery is charged with too much voltage. This causes the electrons to flow from the positive terminal to the negative terminal at an accelerated rate, resulting in a negative charge. This can cause the battery to become damaged or even fail, so it is important to ensure that the voltage being applied to the battery is within the recommended range.
What Are the Benefits of Charging a Battery?
The benefits of charging a battery include increased performance and a longer life. By charging a battery regularly, you can ensure that it is always at its peak performance. This can help to extend the life of the battery, reducing the need to replace it as often. Additionally, charging a battery can help to reduce the amount of energy wasted, as it can be used more efficiently.
Top 6 Frequently Asked Questions
What is a Battery?
A battery is an electrical device that converts stored chemical energy into electrical energy. It consists of one or more cells, each of which contains a positive terminal, or cathode, and a negative terminal, or anode. The terminals are connected to a conductive material such as a metal plate, allowing the flow of electric current. Batteries are used in a variety of devices, from flashlights to cars, as a source of power.
What is a Negative Charge?
A negative charge is an electrical charge that is created when electrons are added to an atom or molecule. It has the opposite effect of a positive charge, which is created when electrons are removed. Negative charges are attracted to positive charges and repel other negative charges.
Are Batteries Negatively Charged?
No, batteries are not negatively charged. Batteries use chemical reactions to create an electrical current, which is composed of positive and negative charges. The positive and negative charges do not have the same properties as a negative charge, so batteries are not negatively charged.
What is the Difference Between a Battery and a Negative Charge?
The main difference between a battery and a negative charge is that a battery is an electrical device that produces power, while a negative charge is an electrical charge that is created when electrons are added to an atom or molecule. Batteries use chemical reactions to create an electrical current, while a negative charge does not produce power.
How Does a Battery Produce Power?
A battery produces power by converting stored chemical energy into electrical energy. The battery is composed of one or more cells, each of which contains a positive terminal, or cathode, and a negative terminal, or anode. When the two terminals are connected to a conductive material such as a metal plate, an electric current is produced. The electric current is then used to power electronic devices.
What is the Purpose of a Battery?
The purpose of a battery is to provide a source of electrical power. Batteries are used in a variety of devices, such as flashlights, computers, and cars, to provide power. Batteries are also used to store energy, such as solar energy, for use at a later time. Finally, batteries can be used to power portable devices, such as cell phones and laptops, which can be used when there is no access to an electrical outlet.
How batteries work – Adam Jacobson
In conclusion, batteries can be both positively and negatively charged, depending on the type of battery. Rechargeable batteries are usually negatively charged, while non-rechargeable batteries are usually positively charged. Ultimately, understanding the basics of battery charge is important for anyone looking to use batteries in their everyday life.